当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Density, Viscosity, and N2O Solubility of Aqueous Solutions of MEA, BmimBF4, and Their Mixtures from 293.15 to 333.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-06-15 , DOI: 10.1021/acs.jced.8b00070 Lingjun Xu 1 , Shujuan Wang 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-06-15 , DOI: 10.1021/acs.jced.8b00070 Lingjun Xu 1 , Shujuan Wang 1
Affiliation
|
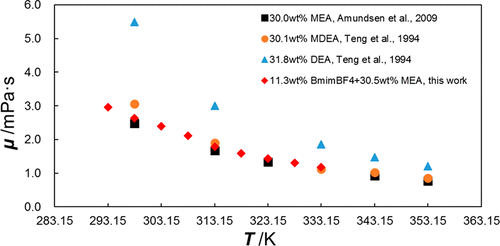
Densities and viscosities of aqueous solutions of ethanolamine (MEA), 1-butyl-3-methylimidazolium tetrafluoroborate (BmimBF4), and their mixtures were measured. The experiments covered the following mole fraction ranges: 1.00–9.38 mol % of BmimBF4 in its aqueous solutions, 3.94–21.97 mol % of MEA in its aqueous solutions, and 1.32–17.01 mol % of MEA + 5.10–34.02 mol % of BmimBF4 in the mixtures. The results are compared with available data in the literature and correlated using a semiempirical formula as functions of the temperature and concentration of MEA and BmimBF4. At the concentration suitable for the CO2 capture, the viscosity of the hybrid absorbent did not significantly increase by the addition of BmimBF4. Moreover, the addition of BmimBF4 reduced the Henry’s law constant, which is significant for reaction kinetics.
中文翻译:

293.15至333.15 K的MEA,BmimBF 4及其混合物的水溶液的密度,粘度和N 2 O溶解度
测量乙醇胺(MEA),1-丁基-3-甲基咪唑鎓四氟硼酸盐(BmimBF 4)及其混合物的水溶液的密度和粘度。实验涵盖以下摩尔分数范围:水溶液中BmimBF 4的浓度为1.00–9.38 mol%,水溶液中MEA的浓度为3.94–21.97 mol%,MEA的浓度为1.32–17.01 mol%+ BmimBF的浓度为5.10–34.02 mol%在混合物中为4。将结果与文献中的可用数据进行比较,并使用半经验公式将其与MEA和BmimBF 4的温度和浓度相关联。在适合CO 2的浓度下通过捕获BmimBF 4,杂化吸收剂的粘度不会显着增加。此外,BmimBF 4的添加降低了亨利定律常数,这对于反应动力学很重要。
更新日期:2018-06-15
中文翻译:

293.15至333.15 K的MEA,BmimBF 4及其混合物的水溶液的密度,粘度和N 2 O溶解度
测量乙醇胺(MEA),1-丁基-3-甲基咪唑鎓四氟硼酸盐(BmimBF 4)及其混合物的水溶液的密度和粘度。实验涵盖以下摩尔分数范围:水溶液中BmimBF 4的浓度为1.00–9.38 mol%,水溶液中MEA的浓度为3.94–21.97 mol%,MEA的浓度为1.32–17.01 mol%+ BmimBF的浓度为5.10–34.02 mol%在混合物中为4。将结果与文献中的可用数据进行比较,并使用半经验公式将其与MEA和BmimBF 4的温度和浓度相关联。在适合CO 2的浓度下通过捕获BmimBF 4,杂化吸收剂的粘度不会显着增加。此外,BmimBF 4的添加降低了亨利定律常数,这对于反应动力学很重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号