当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction Kinetics of CO2 with Monoethanolamine in n-Propanol. 1. Reaction Kinetic Data and Comparison with Existing Rate Law Expressions
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-07-13 , DOI: 10.1021/acs.iecr.8b01482 Louis J. Du Preez 1 , Jakobus P. Barnard 1 , Linda H. Callanan 1 , Johannes H. Knoetze 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-07-13 , DOI: 10.1021/acs.iecr.8b01482 Louis J. Du Preez 1 , Jakobus P. Barnard 1 , Linda H. Callanan 1 , Johannes H. Knoetze 1
Affiliation
|
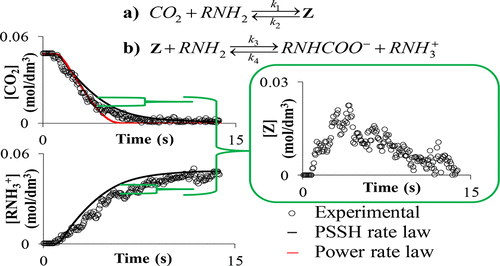
The reaction kinetics of CO2 with monoethanolamine is of industrial significance with respect to both CO2 sequestration applications and characterizing the effective interfacial mass transfer area of packed separation columns. Reaction kinetic data were previously, by necessity, only measured under pseudo-first-order conditions with respect to CO2. Furthermore, mass-transfer limitations encountered by the heterogeneous techniques restricted the validity range of the reaction kinetic models developed from the data. New reaction kinetic data, independent of mass-transfer limitations and outside pseudo-first-order conditions, are presented. The data, collected via a previously developed novel, in situ FTIR technique, were subsequently compared with the predictions of two widely accepted rate expressions, the power rate law and pseudo-steady-state hypothesis (PSSH) rate law. The expressions were modeled on the data using a novel multiobjective goal attainment algorithm also developed in this study. The PSSH rate law predictions were in closer agreement with the data than the power rate law, but both rate expressions were found to be unable to accurately describe the reaction kinetics of CO2, proving that they should be used with caution outside of the pseudo-first-order conditions of their derivation. It was, therefore, concluded that a rate law able to describe the reaction kinetics for all reaction conditions should include the zwitterion reaction intermediate concentration in its fundamentally derived rate expression(s).
中文翻译:

CO 2与单乙醇胺在正丙醇中的反应动力学。1.反应动力学数据及与现有速率定律表达式的比较
关于CO 2螯合应用和表征填充分离塔的有效界面传质面积,CO 2与单乙醇胺的反应动力学具有工业意义。反应动力学数据以前仅根据需要才在伪一级条件下针对CO 2进行测量。。此外,异质技术遇到的传质限制限制了根据数据开发的反应动力学模型的有效性范围。提出了新的反应动力学数据,与传质限制和拟一级条件无关。通过先前开发的新颖的原位FTIR技术收集的数据随后与两种广为接受的费率表达式的预测值进行比较,即功率费率定律和伪稳态假设(PSSH)费率定律。使用也在本研究中开发的新型多目标目标达成算法,根据数据对表达式进行建模。PSSH速率定律的预测与功率定律相比与数据更接近,但是发现两种速率表达式均不能准确描述CO的反应动力学2,证明在推导的伪一阶条件之外应谨慎使用它们。因此,得出结论,能够描述所有反应条件的反应动力学的速率定律应在其基本推导的速率表达式中包括两性离子反应中间体的浓度。
更新日期:2018-07-14
中文翻译:

CO 2与单乙醇胺在正丙醇中的反应动力学。1.反应动力学数据及与现有速率定律表达式的比较
关于CO 2螯合应用和表征填充分离塔的有效界面传质面积,CO 2与单乙醇胺的反应动力学具有工业意义。反应动力学数据以前仅根据需要才在伪一级条件下针对CO 2进行测量。。此外,异质技术遇到的传质限制限制了根据数据开发的反应动力学模型的有效性范围。提出了新的反应动力学数据,与传质限制和拟一级条件无关。通过先前开发的新颖的原位FTIR技术收集的数据随后与两种广为接受的费率表达式的预测值进行比较,即功率费率定律和伪稳态假设(PSSH)费率定律。使用也在本研究中开发的新型多目标目标达成算法,根据数据对表达式进行建模。PSSH速率定律的预测与功率定律相比与数据更接近,但是发现两种速率表达式均不能准确描述CO的反应动力学2,证明在推导的伪一阶条件之外应谨慎使用它们。因此,得出结论,能够描述所有反应条件的反应动力学的速率定律应在其基本推导的速率表达式中包括两性离子反应中间体的浓度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号