当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Improvement of SNAr Reaction Rate by an Electron‐Withdrawing Group in the Crosslinking of DNA Cytosine‐5 Methyltransferase by a Covalent Oligodeoxyribonucleotide Inhibitor
ChemBioChem ( IF 2.6 ) Pub Date : 2018-07-16 , DOI: 10.1002/cbic.201800244 Yukiko Kasai 1 , Kousuke Sato 2 , Shohei Utsumi 1 , Satoshi Ichikawa 1, 3
ChemBioChem ( IF 2.6 ) Pub Date : 2018-07-16 , DOI: 10.1002/cbic.201800244 Yukiko Kasai 1 , Kousuke Sato 2 , Shohei Utsumi 1 , Satoshi Ichikawa 1, 3
Affiliation
|
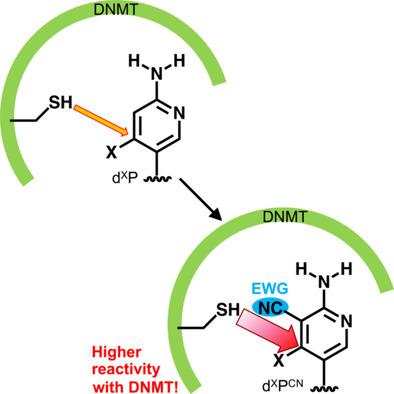
First line of attack: Oligodeoxyribonucleotides containing 2‐amino‐3‐cyano‐4‐halopyridine‐C‐nucleosides (dXPCN) form a complex with DNA cytosine‐5 methyltransferases (DNMTs) through a nucleophilic aromatic substitution (SNAr) reaction. The incorporation of the electron‐withdrawing group (EWG) is effective at increasing the reactivity against nucleophiles of DNMTs, while maintaining high target specificity.

中文翻译:

通过共价寡聚脱氧核糖核苷酸抑制剂的DNA胞嘧啶-5甲基转移酶交联中的吸电子基团提高SNAr反应速率。
攻击的第一行:寡脱氧核糖核苷酸含有2-氨基-3-氰基-4- halopyridine- Ç核苷(d X P CN)通过亲核芳族取代与DNA形成胞嘧啶-5-甲基转移酶(转移酶)的复合物(S Ñ AR)反应。吸电子基团(EWG)的引入可有效提高对DNMT亲核试剂的反应性,同时保持较高的靶标特异性。

更新日期:2018-07-16

中文翻译:

通过共价寡聚脱氧核糖核苷酸抑制剂的DNA胞嘧啶-5甲基转移酶交联中的吸电子基团提高SNAr反应速率。
攻击的第一行:寡脱氧核糖核苷酸含有2-氨基-3-氰基-4- halopyridine- Ç核苷(d X P CN)通过亲核芳族取代与DNA形成胞嘧啶-5-甲基转移酶(转移酶)的复合物(S Ñ AR)反应。吸电子基团(EWG)的引入可有效提高对DNMT亲核试剂的反应性,同时保持较高的靶标特异性。












































 京公网安备 11010802027423号
京公网安备 11010802027423号