当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rational Synthesis of Antiaromatic 5,15‐Dioxaporphyrin and Oxidation into β,β‐Linked Dimers
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-07-13 , DOI: 10.1002/anie.201804648 Akihide Nishiyama 1 , Masaya Fukuda 1 , Shigeki Mori 2 , Ko Furukawa 3 , Heike Fliegl 4 , Hiroyuki Furuta 1, 5 , Soji Shimizu 1, 5
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-07-13 , DOI: 10.1002/anie.201804648 Akihide Nishiyama 1 , Masaya Fukuda 1 , Shigeki Mori 2 , Ko Furukawa 3 , Heike Fliegl 4 , Hiroyuki Furuta 1, 5 , Soji Shimizu 1, 5
Affiliation
|
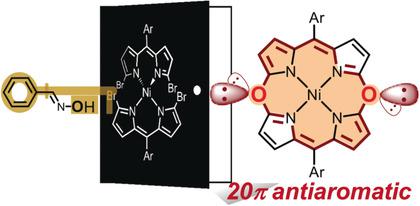
5,15‐Dioxaporphyrin was synthesized for the first time by a nucleophilic aromatic substitution reaction of a nickel bis(α,α′‐dibromodipyrrin) complex with benzaldoxime, followed by an intramolecular annulation of the α‐hydroxy‐substituted intermediate. This unprecedented molecule is a 20π‐electron antiaromatic system, in terms of Hückel's rule of aromaticity, because lone pair electrons of oxygen atoms are incorporated into the 18π‐electron conjugated system of the porphyrin. A theoretical analysis based on the gauge‐including magnetically induced current method confirmed its antiaromaticity and a dominant inner ring pathway for the ring current. The unique reactivity of 5,15‐dioxaporphyrin forming a β,β‐linked dimer upon oxidation was also revealed.
中文翻译:

抗芳香5,15-二氧杂卟啉的合理合成和氧化成β,β-连接的二聚体
通过镍双(α,α'-二溴二吡啶吡啶)络合物与苯甲醛肟的亲核芳香取代反应,然后分子内环氧化α-羟基取代的中间体,首次合成了5,15-二氧杂卟啉。根据Hückel的芳香定律,这种空前的分子是20π电子抗芳香体系,因为氧原子的孤对电子被并入了卟啉的18π电子共轭体系中。基于包括磁感应电流法的量表的理论分析证实了其具有抗芳香性,并且是环电流的主要内环途径。还揭示了5,15-二氧杂卟啉在氧化时形成β,β-连接的二聚体的独特反应性。
更新日期:2018-07-13
中文翻译:

抗芳香5,15-二氧杂卟啉的合理合成和氧化成β,β-连接的二聚体
通过镍双(α,α'-二溴二吡啶吡啶)络合物与苯甲醛肟的亲核芳香取代反应,然后分子内环氧化α-羟基取代的中间体,首次合成了5,15-二氧杂卟啉。根据Hückel的芳香定律,这种空前的分子是20π电子抗芳香体系,因为氧原子的孤对电子被并入了卟啉的18π电子共轭体系中。基于包括磁感应电流法的量表的理论分析证实了其具有抗芳香性,并且是环电流的主要内环途径。还揭示了5,15-二氧杂卟啉在氧化时形成β,β-连接的二聚体的独特反应性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号