当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mixed azido/phenoxido bridged trinuclear Cu(ii) complexes of Mannich bases: Synthesis, structures, magnetic properties and catalytic oxidase activities.
Dalton Transactions ( IF 3.5 ) Pub Date : 2018-07-17 , DOI: 10.1039/c8dt01400k Avijit Das 1 , Kisholoy Bhattacharya 2 , Lakshmi Kanta Das 3 , Sanjib Giri 4 , Ashutosh Ghosh 1
Dalton Transactions ( IF 3.5 ) Pub Date : 2018-07-17 , DOI: 10.1039/c8dt01400k Avijit Das 1 , Kisholoy Bhattacharya 2 , Lakshmi Kanta Das 3 , Sanjib Giri 4 , Ashutosh Ghosh 1
Affiliation
|
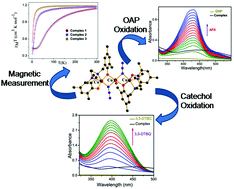
Three similar Mannich base ligands viz. N,N-bis(3,5-dimethyl-2-hydroxybenzyl)-N',N'-dimethyl-1,3-diaminopropane (H2L1), N,N-bis(3,5-dimethyl-2-hydroxybenzyl)-N',N'-dimethyl-1,2-diaminoethane (H2L2) and N,N-bis(3,5-dimethyl-2-hydroxybenzyl)-N',N'-diethyl-1,2-diaminoethane (H2L3) upon reaction with Cu(CH3COO)2·H2O produced dinuclear complexes [Cu2L21-3]. The reaction of each of these isolated dimeric species with Cu(ClO4)2·6H2O and NaN3 resulted in three new trinuclear complexes, [(CuL1)2(μ1,1-N3)2Cu(H2O)]·CH3OH (1), [(CuL2)2(μ1,1-N3)2Cu(H2O)]·CH3OH (2) and [(CuL3)2(μ1,1-N3)2Cu(H2O)]·2CH3OH (3), respectively. The complexes (1-3) have been characterized by elemental analysis and single crystal X-ray diffraction. In all three complexes, the central Cu(ii) ion is coordinated by two terminal [CuL] units through a phenoxido and an azido bridge. These are the first trinuclear Cu(ii) complexes of this type of Mannich base ligands. Magnetic susceptibility measurements showed intramolecular antiferromagnetic interactions with J = -64.42, -9.60 and -4.54 cm-1 for 1, 2 and 3, respectively. All three complexes exhibited catecholase-like and phenoxazinone synthase-like activities towards the aerobic oxidation of 3,5-di-tert-butylcatechol and o-aminophenol, respectively. The turnover numbers (kcat) for the aerobic oxidation of 3,5-di-tert-butylcatechol are 568.8, 542.1 and 500.4 h-1 and those of o-aminophenol are 125.83, 118.9 and 114.7 h-1 for complexes 1-3, respectively. The X-band EPR spectroscopy and estimation of the produced hydrogen peroxide indicated that the aerobic oxidation of 3,5-di-tert-butylcatechol proceeded through the formation of a semiquinonate radical. The mechanism of phenoxazinone synthase-like activities is also proposed for trinuclear Cu(ii) catalysts with the help of mass spectral analysis.
中文翻译:

曼尼希碱的混合叠氮基/苯氧基桥联的三核Cu(ii)配合物:合成,结构,磁性和催化氧化酶活性。
三种相似的曼尼希碱基配体。N,N-双(3,5-二甲基-2-羟基苄基)-N',N'-二甲基-1,3-二氨基丙烷(H2L1),N,N-双(3,5-二甲基-2-羟基苄基) -N',N'-二甲基-1,2-二氨基乙烷(H2L2)和N,N-双(3,5-二甲基-2-羟基苄基)-N',N'-二乙基-1,2-二氨基乙烷(H2L3 )与Cu(CH3COO)2·H2O反应生成双核配合物[Cu2L21-3]。这些分离的二聚体每种与Cu(ClO4)2·6H2O和NaN3的反应产生了三个新的三核络合物[[CuL1)2(μ1,1-N3)2Cu(H2O)]·CH3OH(1),[ (CuL2)2(μ1,1-N3)2Cu(H2O)]·CH3OH(2)和[(CuL3)2(μ1,1-N3)2Cu(H2O)]·2CH3OH(3)。配合物(1-3)已经通过元素分析和单晶X射线衍射表征。在所有三个复合体中,中心的Cu(ii)离子通过苯氧基和叠氮桥由两个末端[CuL]单元进行配位。这些是这种类型的曼尼希碱配体的第一个三核Cu(ii)配合物。磁化率测量表明分子内反铁磁相互作用分别对于1、2和3分别为J = -64.42,-9.60和-4.54 cm-1。这三种复合物对3,5-二叔丁基邻苯二酚和邻氨基苯酚的有氧氧化均表现出儿茶酚酶样和苯恶嗪酮合酶样活性。3,5-二叔丁基邻苯二酚的好氧氧化的周转数(kcat)为568.8、542.1和500.4 h-1,而配合物1-3的邻氨基苯酚的周转数为125.83、118.9和114.7 h-1。分别。X波段EPR光谱和产生的过氧化氢的估计表明3的好氧氧化,5-二叔丁基邻苯二酚通过形成半奎宁酸酯自由基进行。借助质谱分析,还提出了三核Cu(ii)催化剂的苯恶嗪酮合酶样活性机制。
更新日期:2018-06-14
中文翻译:

曼尼希碱的混合叠氮基/苯氧基桥联的三核Cu(ii)配合物:合成,结构,磁性和催化氧化酶活性。
三种相似的曼尼希碱基配体。N,N-双(3,5-二甲基-2-羟基苄基)-N',N'-二甲基-1,3-二氨基丙烷(H2L1),N,N-双(3,5-二甲基-2-羟基苄基) -N',N'-二甲基-1,2-二氨基乙烷(H2L2)和N,N-双(3,5-二甲基-2-羟基苄基)-N',N'-二乙基-1,2-二氨基乙烷(H2L3 )与Cu(CH3COO)2·H2O反应生成双核配合物[Cu2L21-3]。这些分离的二聚体每种与Cu(ClO4)2·6H2O和NaN3的反应产生了三个新的三核络合物[[CuL1)2(μ1,1-N3)2Cu(H2O)]·CH3OH(1),[ (CuL2)2(μ1,1-N3)2Cu(H2O)]·CH3OH(2)和[(CuL3)2(μ1,1-N3)2Cu(H2O)]·2CH3OH(3)。配合物(1-3)已经通过元素分析和单晶X射线衍射表征。在所有三个复合体中,中心的Cu(ii)离子通过苯氧基和叠氮桥由两个末端[CuL]单元进行配位。这些是这种类型的曼尼希碱配体的第一个三核Cu(ii)配合物。磁化率测量表明分子内反铁磁相互作用分别对于1、2和3分别为J = -64.42,-9.60和-4.54 cm-1。这三种复合物对3,5-二叔丁基邻苯二酚和邻氨基苯酚的有氧氧化均表现出儿茶酚酶样和苯恶嗪酮合酶样活性。3,5-二叔丁基邻苯二酚的好氧氧化的周转数(kcat)为568.8、542.1和500.4 h-1,而配合物1-3的邻氨基苯酚的周转数为125.83、118.9和114.7 h-1。分别。X波段EPR光谱和产生的过氧化氢的估计表明3的好氧氧化,5-二叔丁基邻苯二酚通过形成半奎宁酸酯自由基进行。借助质谱分析,还提出了三核Cu(ii)催化剂的苯恶嗪酮合酶样活性机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号