当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Adequate Amount of Acid‐Base Buffer for Electrochemical Investigation of Proton‐Coupled Electron Transfer Reactions
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-06-13 , DOI: 10.1002/slct.201800297 Lijuan Zhang 1 , Xiao Li 1 , Lian Liu 1 , Yanfang Li 1 , Jianguo Wang 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-06-13 , DOI: 10.1002/slct.201800297 Lijuan Zhang 1 , Xiao Li 1 , Lian Liu 1 , Yanfang Li 1 , Jianguo Wang 1
Affiliation
|
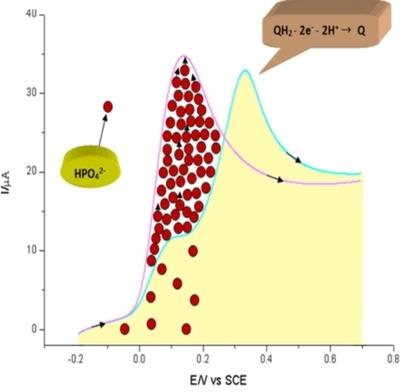
The effective pH occurs near the electrode surface in dilute solutions as protons are released or absorbed during proton‐coupled electron transfer (PCET) reactions. We utilize cyclic voltammetry to study the electrochemical behavior of hydroquinone, p‐benzoquinone, ascorbic acid, and dopamine in phosphate buffer. The effective pH causes peak potential changes and new peak formation. However, the electrochemical behavior of hydroquinone is independent of the buffer when the HPO42−(proton acceptor) concentration is 4.05–6 times greater than that of hydroquinone. Similar results are obtained for quinone in phosphate buffer solution. Dopamine undergoes cyclization during electrochemical oxidation, which also releases protons. Therefore, a high amount of buffer is required. Moreover, the amount of acid‐base buffer strongly affects the electrochemical behaviors of quinone and hydroquinone in acetonitrile. Our results provide a fundamental guide for determining the adequate amount of buffer for the electrochemical investigation of PCET reactions.
中文翻译:

足够量的酸碱缓冲液用于质子偶联电子转移反应的电化学研究
当质子耦合电子转移(PCET)反应过程中释放或吸收质子时,有效pH值将在稀溶液中的电极表面附近发生。我们利用循环伏安法研究了氢醌,对苯醌,抗坏血酸和多巴胺在磷酸盐缓冲液中的电化学行为。有效pH导致峰电位变化和新的峰形成。但是,当HPO 4 2-时,对苯二酚的电化学行为与缓冲液无关(质子受体)浓度是对苯二酚浓度的4.05–6倍。磷酸盐缓冲溶液中的醌获得相似的结果。多巴胺在电化学氧化过程中会发生环化反应,这也会释放质子。因此,需要大量的缓冲区。此外,酸碱缓冲液的量强烈影响醌和对苯二酚在乙腈中的电化学行为。我们的结果为确定用于PCET反应电化学研究的缓冲液的适当量提供了基本指导。
更新日期:2018-06-13
中文翻译:

足够量的酸碱缓冲液用于质子偶联电子转移反应的电化学研究
当质子耦合电子转移(PCET)反应过程中释放或吸收质子时,有效pH值将在稀溶液中的电极表面附近发生。我们利用循环伏安法研究了氢醌,对苯醌,抗坏血酸和多巴胺在磷酸盐缓冲液中的电化学行为。有效pH导致峰电位变化和新的峰形成。但是,当HPO 4 2-时,对苯二酚的电化学行为与缓冲液无关(质子受体)浓度是对苯二酚浓度的4.05–6倍。磷酸盐缓冲溶液中的醌获得相似的结果。多巴胺在电化学氧化过程中会发生环化反应,这也会释放质子。因此,需要大量的缓冲区。此外,酸碱缓冲液的量强烈影响醌和对苯二酚在乙腈中的电化学行为。我们的结果为确定用于PCET反应电化学研究的缓冲液的适当量提供了基本指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号