当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Picosecond Pulse Radiolysis Study on the Radiation-Induced Reactions in Neat Tributyl Phosphate
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-07-03 , DOI: 10.1021/acs.jpcb.8b03715 Furong Wang 1 , Gregory P. Horne 2 , Pascal Pernot 1 , Pierre Archirel 1 , Mehran Mostafavi 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-07-03 , DOI: 10.1021/acs.jpcb.8b03715 Furong Wang 1 , Gregory P. Horne 2 , Pascal Pernot 1 , Pierre Archirel 1 , Mehran Mostafavi 1
Affiliation
|
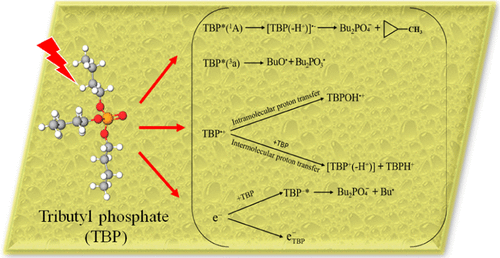
The ultrafast radiolytic behavior of tributyl phosphate, TBP, has been investigated using 7 ps electron pulses with 7 MeV kinetic energy, from which two key species have been observed and characterized: the TBP solvated electron (eTBP–) and the TBP triplet excited state TBP* (3a) or its fragmentation products. The eTBP– exhibits a broad absorption band in the visible and near-infrared (NIR) spectrum, with a maximum beyond our 1500 nm detection limit. Nitromethane was used to scavenge eTBP– to confirm its absorption spectrum and to determine its associated rate coefficient, 1.0 × 1010 M–1 s–1. The electron’s molar extinction coefficients were found by an isosbestic method using biphenyl as a solvated electron scavenger. The time-dependent radiolytic yield of eTBP– was also determined directly from 7 ps to 7 ns and compared with those in water, tetrahydrofuran, and diethyl carbonate. In less than 10 ns, the decay is not due to the reaction with other solvent molecules and is instead predominantly due to the reactions with cations issued from the proton transfer by the TBP radical cation (TBP•+). In addition to eTBP–, another absorption band, stable up to 7 ns, was identified in the visible range. This has been attributed mainly to the TBP triplet excited state, TBP*(3a), by a combination of molecular modeling methodologies. Interestingly, we did not observe any absorption band in the visible nor in the NIR range arising from TBP•+. Calculations suggest that TBP•+ undergoes rapid proton transfer to yield a UV-absorbing species, TBP(−H+). Experimental results and supporting molecular simulations provide detailed identification of the earliest species yielded from the radiolysis of neat TBP.
中文翻译:

皮秒磷酸三丁酯辐射诱导反应的皮秒脉冲辐解研究
使用具有7 MeV动能的7 ps电子脉冲研究了磷酸三丁酯TBP的超快辐射行为,从中观察并表征了两个关键物质:TBP溶剂化电子(e TBP –)和TBP三重激发态TBP *(3 a)或其碎片产物。e TBP –在可见光和近红外(NIR)光谱中显示出较宽的吸收带,其最大值超出了我们的1500 nm检测极限。硝基甲烷用于清除TBP –确认其吸收光谱并确定其相关的速率系数1.0×10 10 M –1 s –1。电子的摩尔消光系数是通过使用联苯作为溶剂化电子清除剂的等吸收方法发现的。e TBP –随时间变化的辐射产率也直接在7 ps至7 ns范围内测定,并与水,四氢呋喃和碳酸二乙酯中的产率进行比较。在不到10 ns的时间内,衰变不是由于与其他溶剂分子的反应,而是主要是由于与TBP自由基阳离子(TBP •+)的质子转移所产生的阳离子发生反应。除了e TBP –之外,在可见光范围内还发现了另一个吸收带,该吸收带稳定至7 ns。这主要归因于TBP三重态激发态TBP *(3a),结合分子建模方法。有趣的是,我们没有观察到由TBP •+引起的在可见光或NIR范围内的吸收带。计算表明,TBP •+经历了快速的质子转移,从而产生了吸收紫外线的物质TBP(-H +)。实验结果和支持的分子模拟提供了对纯TBP辐射分解产生的最早物质的详细鉴定。
更新日期:2018-07-04
中文翻译:

皮秒磷酸三丁酯辐射诱导反应的皮秒脉冲辐解研究
使用具有7 MeV动能的7 ps电子脉冲研究了磷酸三丁酯TBP的超快辐射行为,从中观察并表征了两个关键物质:TBP溶剂化电子(e TBP –)和TBP三重激发态TBP *(3 a)或其碎片产物。e TBP –在可见光和近红外(NIR)光谱中显示出较宽的吸收带,其最大值超出了我们的1500 nm检测极限。硝基甲烷用于清除TBP –确认其吸收光谱并确定其相关的速率系数1.0×10 10 M –1 s –1。电子的摩尔消光系数是通过使用联苯作为溶剂化电子清除剂的等吸收方法发现的。e TBP –随时间变化的辐射产率也直接在7 ps至7 ns范围内测定,并与水,四氢呋喃和碳酸二乙酯中的产率进行比较。在不到10 ns的时间内,衰变不是由于与其他溶剂分子的反应,而是主要是由于与TBP自由基阳离子(TBP •+)的质子转移所产生的阳离子发生反应。除了e TBP –之外,在可见光范围内还发现了另一个吸收带,该吸收带稳定至7 ns。这主要归因于TBP三重态激发态TBP *(3a),结合分子建模方法。有趣的是,我们没有观察到由TBP •+引起的在可见光或NIR范围内的吸收带。计算表明,TBP •+经历了快速的质子转移,从而产生了吸收紫外线的物质TBP(-H +)。实验结果和支持的分子模拟提供了对纯TBP辐射分解产生的最早物质的详细鉴定。










































 京公网安备 11010802027423号
京公网安备 11010802027423号