Chem ( IF 19.1 ) Pub Date : 2018-06-19 , DOI: 10.1016/j.chempr.2018.05.006 Xiaoju Cui , Haobo Li , Yan Wang , Yuanli Hu , Lei Hua , Haiyang Li , Xiuwen Han , Qingfei Liu , Fan Yang , Limin He , Xiaoqi Chen , Qingyun Li , Jianping Xiao , Dehui Deng , Xinhe Bao
|
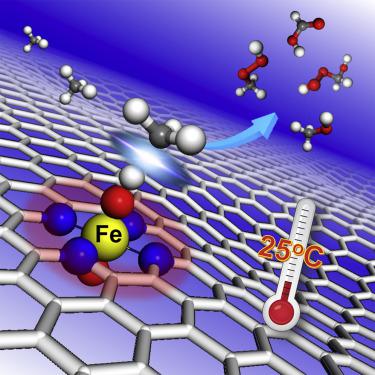
Direct conversion of methane to high-value-added chemicals is a major challenge in catalysis, which usually requires high-energy input to overcome the reaction barrier. We report that graphene-confined single Fe atoms can be used as an efficient non-precious catalyst to directly convert methane to C1 oxygenated products at room temperature. A series of graphene-confined 3d transition metals (Mn, Fe, Co, Ni, and Cu) were screened, yet only single Fe atoms could catalyze the methane conversion. Combining in operando time-of-flight mass spectrometry, 13C nuclear magnetic resonance, and density functional theory calculations, we found that methane conversion proceeds on the O–FeN4–O active site along a radical pathway to produce CH3OH and CH3OOH first, and then the generated CH3OH can be further catalyzed to form HOCH2OOH and HCOOH at room temperature.
中文翻译:

石墨烯限制的单铁原子在室温下进行甲烷转化
甲烷直接转化为高附加值化学品是催化的主要挑战,通常需要高能量输入才能克服反应障碍。我们报道石墨烯限制的单个Fe原子可以用作有效的非贵金属催化剂,在室温下将甲烷直接转化为C1氧化产物。筛选了一系列石墨烯限制的3d过渡金属(Mn,Fe,Co,Ni和Cu),但只有单个Fe原子可以催化甲烷转化。结合飞行时间质谱,13 C核磁共振和密度泛函理论计算,我们发现甲烷的转化沿自由基途径在O–FeN 4 –O活性位点发生,从而生成CH 3首先是OH和CH 3 OOH,然后可以在室温下进一步催化生成的CH 3 OH形成HOCH 2 OOH和HCOOH。











































 京公网安备 11010802027423号
京公网安备 11010802027423号