当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Altering Copper‐Catalyzed A3 Couplings by Mechanochemistry: One‐Pot Synthesis of 1,4‐Diamino‐2‐butynes from Aldehydes, Amines, and Calcium Carbide
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-07-13 , DOI: 10.1002/anie.201805505 Mathias Turberg 1 , Karen J. Ardila‐Fierro 1 , Carsten Bolm 1 , José G. Hernández 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-07-13 , DOI: 10.1002/anie.201805505 Mathias Turberg 1 , Karen J. Ardila‐Fierro 1 , Carsten Bolm 1 , José G. Hernández 1
Affiliation
|
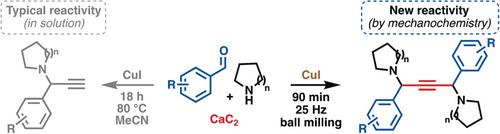
The ability of mechanochemistry to alter established chemical selectivity is demonstrated. A copper(I)‐catalyzed mechanochemical aldehyde/alkyne/amine coupling using calcium carbide as the acetylene source provides selective access to 1,4‐diamino‐2‐butynes, which contrasts classical approaches that provide propargylamine‐type products. Solventless milling conditions were found to be essential to unmask A3 coupling products with new compositions.
中文翻译:

通过机械化学改变铜催化的A3偶联:由醛,胺和碳化钙一锅法合成1,4-二氨基-2-丁炔
证明了机械化学改变既定化学选择性的能力。使用碳化钙作为乙炔源的铜(I)催化的机械化学醛/炔/胺偶联可选择性地获得1,4-二氨基-2-丁炔,这与提供炔丙基胺类产品的传统方法形成了鲜明对比。发现无溶剂的研磨条件对于 用新的组合物掩盖A 3偶联产物是必不可少的。
更新日期:2018-07-13
中文翻译:

通过机械化学改变铜催化的A3偶联:由醛,胺和碳化钙一锅法合成1,4-二氨基-2-丁炔
证明了机械化学改变既定化学选择性的能力。使用碳化钙作为乙炔源的铜(I)催化的机械化学醛/炔/胺偶联可选择性地获得1,4-二氨基-2-丁炔,这与提供炔丙基胺类产品的传统方法形成了鲜明对比。发现无溶剂的研磨条件对于 用新的组合物掩盖A 3偶联产物是必不可少的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号