当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
“Back-to-Front” Indole Synthesis Using Silver(I) Catalysis: Unexpected C-3 Pyrrole Activation Mode Supported by DFT
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-06-13 00:00:00 , DOI: 10.1021/acscatal.8b00745 Aimee K. Clarke 1 , Jason M. Lynam 1 , Richard J. K. Taylor 1 , William P. Unsworth 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-06-13 00:00:00 , DOI: 10.1021/acscatal.8b00745 Aimee K. Clarke 1 , Jason M. Lynam 1 , Richard J. K. Taylor 1 , William P. Unsworth 1
Affiliation
|
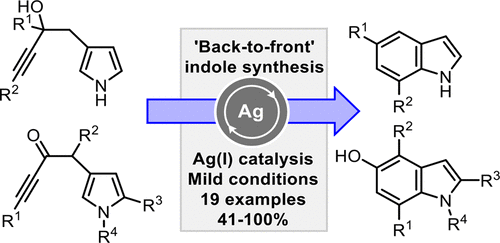
An efficient silver(I)-catalyzed method is reported for the synthesis of substituted indoles, most notably 5-hydroxy-derivatives, via π-acidic alkyne activation. Most methods for the preparation of indoles involve annulation of a benzene precursor, but the method reported herein is unusual in that pyrrole precursors are used. Density Functional Theory (DFT) studies suggest that these reactions proceed via initial activation of the pyrrole C-3 position before undergoing subsequent rearrangement, contradicting the conventional wisdom that pyrroles are more nucleophilic through C-2.
中文翻译:

使用银(I)催化“从前到后”合成吲哚:DFT支持意外的C-3吡咯活化方式
据报道,一种有效的银(I)催化方法可通过π-酸性炔烃活化来合成取代的吲哚,最著名的是5-羟基衍生物。用于制备吲哚的大多数方法涉及苯前体的环化,但是本文报道的方法是不寻常的,因为使用了吡咯前体。密度泛函理论(DFT)研究表明,这些反应是通过吡咯C-3位置的初始活化进行的,然后进行后续重排,这与传统的观点认为吡咯通过C-2更具亲核性有关。
更新日期:2018-06-13
中文翻译:

使用银(I)催化“从前到后”合成吲哚:DFT支持意外的C-3吡咯活化方式
据报道,一种有效的银(I)催化方法可通过π-酸性炔烃活化来合成取代的吲哚,最著名的是5-羟基衍生物。用于制备吲哚的大多数方法涉及苯前体的环化,但是本文报道的方法是不寻常的,因为使用了吡咯前体。密度泛函理论(DFT)研究表明,这些反应是通过吡咯C-3位置的初始活化进行的,然后进行后续重排,这与传统的观点认为吡咯通过C-2更具亲核性有关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号