当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stability of Biological Membranes upon Mechanical Indentation
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-07-05 , DOI: 10.1021/acs.jpcb.8b01861 Florian Franz 1 , Camilo Aponte-Santamaría 2, 3 , Csaba Daday 1 , Vedran Miletić 1 , Frauke Gräter 1, 3
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-07-05 , DOI: 10.1021/acs.jpcb.8b01861 Florian Franz 1 , Camilo Aponte-Santamaría 2, 3 , Csaba Daday 1 , Vedran Miletić 1 , Frauke Gräter 1, 3
Affiliation
|
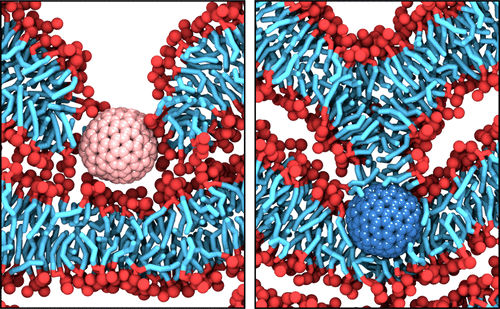
Mechanical perturbations are ubiquitous in living cells, and many biological functions are dependent on the mechanical response of lipid membranes. Recent force-spectroscopy studies have captured the stepwise fracture of stacks of bilayers, avoiding substrate effects. However, the effect of stacking bilayers, as well as the exact molecular mechanism of the fracture process, is unknown. Here, we use atomistic and coarse-grained force-clamp molecular dynamics simulation to assess the effects of mechanical indentation on stacked and single bilayers. Our simulations show that the rupture process obeys the laws of force-activated barrier crossing, and stacking multiple membranes stabilizes them. The rupture times follow a log-normal distribution which allows the interpretation of membrane rupture as a pore-growth process. Indenter hydrophobicity determines the type of pore formation, the preferred dwelling region, and the resistance of the bilayer against rupture. Our results provide a better understanding of the nanomechanics underlying the plastic rupture of lipid membranes.
中文翻译:

机械压痕后生物膜的稳定性
机械干扰在活细胞中无处不在,许多生物学功能取决于脂质膜的机械反应。最近的力谱研究已经捕获了双层堆叠的逐步断裂,从而避免了底物的影响。但是,双层堆积的作用以及断裂过程的确切分子机理尚不清楚。在这里,我们使用原子和粗粒度的力夹分子动力学模拟来评估机械压痕对堆叠和单双层的影响。我们的模拟表明,破裂过程遵循力激活屏障越过的规律,并且堆叠多个膜可以使它们稳定。破裂时间遵循对数正态分布,这允许将膜破裂解释为孔隙生长过程。压头疏水性决定了孔形成的类型,优选的驻留区域以及双层的抗破裂性。我们的结果提供了对脂质膜可塑性破裂的纳米力学的更好理解。
更新日期:2018-07-08
中文翻译:

机械压痕后生物膜的稳定性
机械干扰在活细胞中无处不在,许多生物学功能取决于脂质膜的机械反应。最近的力谱研究已经捕获了双层堆叠的逐步断裂,从而避免了底物的影响。但是,双层堆积的作用以及断裂过程的确切分子机理尚不清楚。在这里,我们使用原子和粗粒度的力夹分子动力学模拟来评估机械压痕对堆叠和单双层的影响。我们的模拟表明,破裂过程遵循力激活屏障越过的规律,并且堆叠多个膜可以使它们稳定。破裂时间遵循对数正态分布,这允许将膜破裂解释为孔隙生长过程。压头疏水性决定了孔形成的类型,优选的驻留区域以及双层的抗破裂性。我们的结果提供了对脂质膜可塑性破裂的纳米力学的更好理解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号