当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
1-Butanol as a Solvent for Efficient Extraction of Polar Compounds from Aqueous Medium: Theoretical and Practical Aspects
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-07-02 , DOI: 10.1021/acs.jpcb.8b02877 Gerhard König 1, 2 , Manfred T. Reetz 1, 3 , Walter Thiel 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-07-02 , DOI: 10.1021/acs.jpcb.8b02877 Gerhard König 1, 2 , Manfred T. Reetz 1, 3 , Walter Thiel 1
Affiliation
|
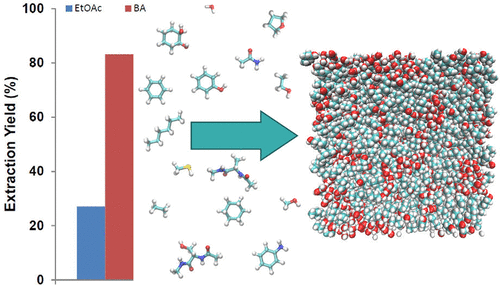
The extraction of polar molecules from aqueous solution is a challenging task in organic synthesis. 1-Butanol has been used sporadically as an eluent for polar molecules, but it is unclear which molecular features drive its efficiency. Here, we employ free energy simulations to study the partitioning of 15 solutes between water and 1-butanol. The simulations demonstrate that the high affinity of polar molecules to the wet 1-butanol phase is associated with its nanostructure. Small inverse micelles of water are able to accommodate polar solutes and locally mimic an aqueous environment. We verify the simulations based on partition coefficients between water and 1-octanol, and include a blind prediction of the water/1-butanol partition coefficient of cyclohexane-1,2-diol. The calculations are in excellent agreement with experiment, reaching root-mean-square deviations below 0.7 kcal/mol. Actual extractions of cyclohexane-1,2-diol from buffer solutions that mimic cell lysates and suspensions in biocatalytic reactions further exemplify our findings. The yields highlight that extractions with 1-butanol can be significantly more efficient than the conventional protocol based on ethyl acetate.
中文翻译:

1-丁醇作为从水性介质中高效萃取极性化合物的溶剂:理论和实践方面
从水溶液中提取极性分子是有机合成中的一项艰巨任务。1-丁醇已偶发用作极性分子的洗脱液,但尚不清楚哪些分子特征可提高其效率。在这里,我们采用自由能模拟来研究15种溶质在水和1-丁醇之间的分配。模拟表明极性分子对湿的1-丁醇相的高亲和力与其纳米结构有关。小的反胶束水可以容纳极性溶质并局部模拟水性环境。我们验证了基于水和1-辛醇之间分配系数的模拟,并包括对环己烷-1,2-二醇的水/ 1-丁醇分配系数的盲目预测。计算结果与实验非常吻合,达到均方根偏差低于0.7 kcal / mol。从模拟细胞裂解液和生物催化反应中的悬浮液的缓冲溶液中实际提取环己烷-1,2-二醇的方法进一步证明了我们的发现。产率突出表明,用1-丁醇萃取比传统的基于乙酸乙酯的实验方案效率更高。
更新日期:2018-07-02
中文翻译:

1-丁醇作为从水性介质中高效萃取极性化合物的溶剂:理论和实践方面
从水溶液中提取极性分子是有机合成中的一项艰巨任务。1-丁醇已偶发用作极性分子的洗脱液,但尚不清楚哪些分子特征可提高其效率。在这里,我们采用自由能模拟来研究15种溶质在水和1-丁醇之间的分配。模拟表明极性分子对湿的1-丁醇相的高亲和力与其纳米结构有关。小的反胶束水可以容纳极性溶质并局部模拟水性环境。我们验证了基于水和1-辛醇之间分配系数的模拟,并包括对环己烷-1,2-二醇的水/ 1-丁醇分配系数的盲目预测。计算结果与实验非常吻合,达到均方根偏差低于0.7 kcal / mol。从模拟细胞裂解液和生物催化反应中的悬浮液的缓冲溶液中实际提取环己烷-1,2-二醇的方法进一步证明了我们的发现。产率突出表明,用1-丁醇萃取比传统的基于乙酸乙酯的实验方案效率更高。









































 京公网安备 11010802027423号
京公网安备 11010802027423号