Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure of the µ-opioid receptor–Gi protein complex
Nature ( IF 50.5 ) Pub Date : 2018-06-01 , DOI: 10.1038/s41586-018-0219-7 Antoine Koehl 1 , Hongli Hu 1, 2 , Shoji Maeda 2 , Yan Zhang 1, 2 , Qianhui Qu 1, 2 , Joseph M Paggi 1, 2, 3, 4 , Naomi R Latorraca 1, 2, 3, 4, 5 , Daniel Hilger 2 , Roger Dawson 6 , Hugues Matile 6 , Gebhard F X Schertler 7, 8 , Sebastien Granier 9 , William I Weis 1, 2 , Ron O Dror 1, 2, 3, 4, 5 , Aashish Manglik 10, 11 , Georgios Skiniotis 1, 2 , Brian K Kobilka 2
Nature ( IF 50.5 ) Pub Date : 2018-06-01 , DOI: 10.1038/s41586-018-0219-7 Antoine Koehl 1 , Hongli Hu 1, 2 , Shoji Maeda 2 , Yan Zhang 1, 2 , Qianhui Qu 1, 2 , Joseph M Paggi 1, 2, 3, 4 , Naomi R Latorraca 1, 2, 3, 4, 5 , Daniel Hilger 2 , Roger Dawson 6 , Hugues Matile 6 , Gebhard F X Schertler 7, 8 , Sebastien Granier 9 , William I Weis 1, 2 , Ron O Dror 1, 2, 3, 4, 5 , Aashish Manglik 10, 11 , Georgios Skiniotis 1, 2 , Brian K Kobilka 2
Affiliation
|
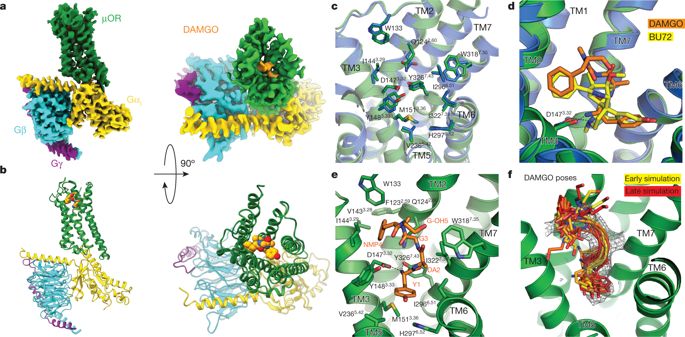
The μ-opioid receptor (μOR) is a G-protein-coupled receptor (GPCR) and the target of most clinically and recreationally used opioids. The induced positive effects of analgesia and euphoria are mediated by μOR signalling through the adenylyl cyclase-inhibiting heterotrimeric G protein Gi. Here we present the 3.5 Å resolution cryo-electron microscopy structure of the μOR bound to the agonist peptide DAMGO and nucleotide-free Gi. DAMGO occupies the morphinan ligand pocket, with its N terminus interacting with conserved receptor residues and its C terminus engaging regions important for opioid-ligand selectivity. Comparison of the μOR–Gi complex to previously determined structures of other GPCRs bound to the stimulatory G protein Gs reveals differences in the position of transmembrane receptor helix 6 and in the interactions between the G protein α-subunit and the receptor core. Together, these results shed light on the structural features that contribute to the Gi protein-coupling specificity of the µOR.A cryo-electron structure of the µ-opioid receptor in complex with the peptide agonist DAMGO and the inhibitory G protein Gi reveals structural determinants of its G protein-binding specificity.
中文翻译:

µ-阿片受体-Gi 蛋白复合物的结构
μ-阿片受体 (μOR) 是一种 G 蛋白偶联受体 (GPCR),也是大多数临床和娱乐用途阿片类药物的靶标。镇痛和欣快感的诱导积极作用是通过腺苷酸环化酶抑制异三聚体 G 蛋白 Gi 的 μOR 信号介导的。在这里,我们展示了与激动剂肽 DAMGO 和无核苷酸 Gi 结合的 μOR 的 3.5 Å 分辨率冷冻电子显微镜结构。 DAMGO 占据吗啡喃配体口袋,其 N 末端与保守的受体残基相互作用,其 C 末端接合对阿片类药物配体选择性很重要的区域。将μOR-Gi复合物与先前确定的与刺激性G蛋白Gs结合的其他GPCR的结构进行比较,揭示了跨膜受体螺旋6的位置以及G蛋白α亚基与受体核心之间的相互作用的差异。总之,这些结果揭示了有助于 µOR 的 Gi 蛋白偶联特异性的结构特征。 µ-阿片受体与肽激动剂 DAMGO 和抑制性 G 蛋白 Gi 复合的冷冻电子结构揭示了结构决定因素其 G 蛋白结合特异性。
更新日期:2018-06-01
中文翻译:

µ-阿片受体-Gi 蛋白复合物的结构
μ-阿片受体 (μOR) 是一种 G 蛋白偶联受体 (GPCR),也是大多数临床和娱乐用途阿片类药物的靶标。镇痛和欣快感的诱导积极作用是通过腺苷酸环化酶抑制异三聚体 G 蛋白 Gi 的 μOR 信号介导的。在这里,我们展示了与激动剂肽 DAMGO 和无核苷酸 Gi 结合的 μOR 的 3.5 Å 分辨率冷冻电子显微镜结构。 DAMGO 占据吗啡喃配体口袋,其 N 末端与保守的受体残基相互作用,其 C 末端接合对阿片类药物配体选择性很重要的区域。将μOR-Gi复合物与先前确定的与刺激性G蛋白Gs结合的其他GPCR的结构进行比较,揭示了跨膜受体螺旋6的位置以及G蛋白α亚基与受体核心之间的相互作用的差异。总之,这些结果揭示了有助于 µOR 的 Gi 蛋白偶联特异性的结构特征。 µ-阿片受体与肽激动剂 DAMGO 和抑制性 G 蛋白 Gi 复合的冷冻电子结构揭示了结构决定因素其 G 蛋白结合特异性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号