Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cryo-EM structure of human rhodopsin bound to an inhibitory G protein
Nature ( IF 50.5 ) Pub Date : 2018-06-01 , DOI: 10.1038/s41586-018-0215-y Yanyong Kang 1 , Oleg Kuybeda 2 , Parker W de Waal 1 , Somnath Mukherjee 3 , Ned Van Eps 4 , Przemyslaw Dutka 3, 5 , X Edward Zhou 1 , Alberto Bartesaghi 2 , Satchal Erramilli 3 , Takefumi Morizumi 4 , Xin Gu 1 , Yanting Yin 1 , Ping Liu 6, 7 , Yi Jiang 7 , Xing Meng 8 , Gongpu Zhao 8 , Karsten Melcher 1 , Oliver P Ernst 4, 9 , Anthony A Kossiakoff 3, 10 , Sriram Subramaniam 2, 11 , H Eric Xu 1, 7
Nature ( IF 50.5 ) Pub Date : 2018-06-01 , DOI: 10.1038/s41586-018-0215-y Yanyong Kang 1 , Oleg Kuybeda 2 , Parker W de Waal 1 , Somnath Mukherjee 3 , Ned Van Eps 4 , Przemyslaw Dutka 3, 5 , X Edward Zhou 1 , Alberto Bartesaghi 2 , Satchal Erramilli 3 , Takefumi Morizumi 4 , Xin Gu 1 , Yanting Yin 1 , Ping Liu 6, 7 , Yi Jiang 7 , Xing Meng 8 , Gongpu Zhao 8 , Karsten Melcher 1 , Oliver P Ernst 4, 9 , Anthony A Kossiakoff 3, 10 , Sriram Subramaniam 2, 11 , H Eric Xu 1, 7
Affiliation
|
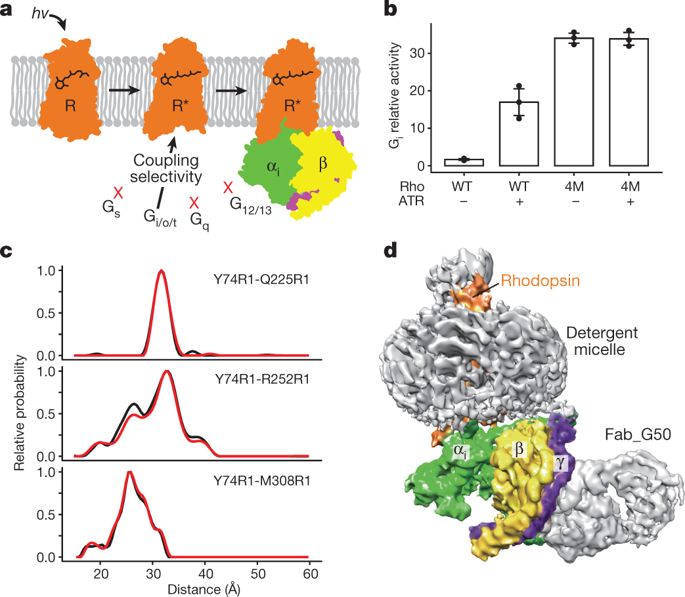
G-protein-coupled receptors comprise the largest family of mammalian transmembrane receptors. They mediate numerous cellular pathways by coupling with downstream signalling transducers, including the hetrotrimeric G proteins Gs (stimulatory) and Gi (inhibitory) and several arrestin proteins. The structural mechanisms that define how G-protein-coupled receptors selectively couple to a specific type of G protein or arrestin remain unknown. Here, using cryo-electron microscopy, we show that the major interactions between activated rhodopsin and Gi are mediated by the C-terminal helix of the Gi α-subunit, which is wedged into the cytoplasmic cavity of the transmembrane helix bundle and directly contacts the amino terminus of helix 8 of rhodopsin. Structural comparisons of inactive, Gi-bound and arrestin-bound forms of rhodopsin with inactive and Gs-bound forms of the β2-adrenergic receptor provide a foundation to understand the unique structural signatures that are associated with the recognition of Gs, Gi and arrestin by activated G-protein-coupled receptors.The cryo-electron microscopy structure of human rhodopsin bound to the inhibitory Gi protein-coupled receptor provides insights into ligand–receptor–G-protein interactions.
中文翻译:

人视紫红质与抑制性 G 蛋白结合的冷冻电镜结构
G 蛋白偶联受体包括最大的哺乳动物跨膜受体家族。它们通过与下游信号传导转导器耦合来介导多种细胞通路,包括异源三聚体 G 蛋白 Gs(刺激性)和 Gi(抑制性)以及几种视紫红质抑制蛋白。定义 G 蛋白偶联受体如何选择性地与特定类型的 G 蛋白或抑制蛋白偶联的结构机制仍然未知。在这里,使用冷冻电子显微镜,我们发现活化的视紫红质和 Gi 之间的主要相互作用是由 Gi α 亚基的 C 端螺旋介导的,该螺旋楔入跨膜螺旋束的细胞质腔并直接接触视紫红质螺旋8的氨基末端。将无活性、Gi 结合和视紫红质抑制蛋白结合形式与无活性和 Gs 结合形式的 β2-肾上腺素能受体进行结构比较,为了解与 Gs、Gi 和视紫红质抑制蛋白识别相关的独特结构特征奠定了基础。激活的 G 蛋白偶联受体。与抑制性 Gi 蛋白偶联受体结合的人视紫红质的冷冻电子显微镜结构提供了对配体-受体-G 蛋白相互作用的深入了解。
更新日期:2018-06-01
中文翻译:

人视紫红质与抑制性 G 蛋白结合的冷冻电镜结构
G 蛋白偶联受体包括最大的哺乳动物跨膜受体家族。它们通过与下游信号传导转导器耦合来介导多种细胞通路,包括异源三聚体 G 蛋白 Gs(刺激性)和 Gi(抑制性)以及几种视紫红质抑制蛋白。定义 G 蛋白偶联受体如何选择性地与特定类型的 G 蛋白或抑制蛋白偶联的结构机制仍然未知。在这里,使用冷冻电子显微镜,我们发现活化的视紫红质和 Gi 之间的主要相互作用是由 Gi α 亚基的 C 端螺旋介导的,该螺旋楔入跨膜螺旋束的细胞质腔并直接接触视紫红质螺旋8的氨基末端。将无活性、Gi 结合和视紫红质抑制蛋白结合形式与无活性和 Gs 结合形式的 β2-肾上腺素能受体进行结构比较,为了解与 Gs、Gi 和视紫红质抑制蛋白识别相关的独特结构特征奠定了基础。激活的 G 蛋白偶联受体。与抑制性 Gi 蛋白偶联受体结合的人视紫红质的冷冻电子显微镜结构提供了对配体-受体-G 蛋白相互作用的深入了解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号