Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Cdk9–PP1 switch regulates the elongation–termination transition of RNA polymerase II
Nature ( IF 50.5 ) Pub Date : 2018-06-01 , DOI: 10.1038/s41586-018-0214-z Pabitra K Parua 1 , Gregory T Booth 2 , Miriam Sansó 1, 3 , Bradley Benjamin 1 , Jason C Tanny 4 , John T Lis 2 , Robert P Fisher 1
Nature ( IF 50.5 ) Pub Date : 2018-06-01 , DOI: 10.1038/s41586-018-0214-z Pabitra K Parua 1 , Gregory T Booth 2 , Miriam Sansó 1, 3 , Bradley Benjamin 1 , Jason C Tanny 4 , John T Lis 2 , Robert P Fisher 1
Affiliation
|
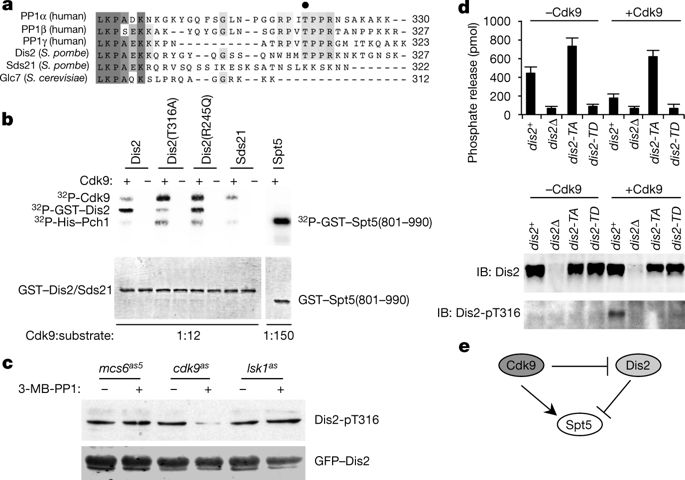
The end of the RNA polymerase II (Pol II) transcription cycle is strictly regulated to prevent interference between neighbouring genes and to safeguard transcriptome integrity1. The accumulation of Pol II downstream of the cleavage and polyadenylation signal can facilitate the recruitment of factors involved in mRNA 3′-end formation and termination2, but how this sequence is initiated remains unclear. In a chemical–genetic screen, human protein phosphatase 1 (PP1) isoforms were identified as substrates of positive transcription elongation factor b (P-TEFb), also known as the cyclin-dependent kinase 9 (Cdk9)–cyclin T1 (CycT1) complex3. Here we show that Cdk9 and PP1 govern phosphorylation of the conserved elongation factor Spt5 in the fission yeast Schizosaccharomyces pombe. Cdk9 phosphorylates both Spt5 and a negative regulatory site on the PP1 isoform Dis24. Sites targeted by Cdk9 in the Spt5 carboxy-terminal domain can be dephosphorylated by Dis2 in vitro, and dis2 mutations retard Spt5 dephosphorylation after inhibition of Cdk9 in vivo. Chromatin immunoprecipitation and sequencing analysis indicates that Spt5 is dephosphorylated as transcription complexes traverse the cleavage and polyadenylation signal, concomitant with the accumulation of Pol II phosphorylated at residue Ser2 of the carboxy-terminal domain consensus heptad repeat5. A conditionally lethal Dis2-inactivating mutation attenuates the drop in Spt5 phosphorylation on chromatin, promotes transcription beyond the normal termination zone (as detected by precision run-on transcription and sequencing6) and is genetically suppressed by the ablation of Cdk9 target sites in Spt5. These results suggest that the transition of Pol II from elongation to termination coincides with a Dis2-dependent reversal of Cdk9 signalling—a switch that is analogous to a Cdk1–PP1 circuit that controls mitotic progression4.The kinase Cdk9 and the phosphatase Dis2 regulate the termination of transcription in fission yeast in part by controlling the phosphorylation state of the elongation factor Spt5.
中文翻译:

Cdk9-PP1 开关调节 RNA 聚合酶 II 的延伸-终止转变
RNA 聚合酶 II (Pol II) 转录周期的结束受到严格监管,以防止相邻基因之间的干扰并保护转录组的完整性 1。Pol II 在切割和聚腺苷酸化信号下游的积累可以促进参与 mRNA 3'-末端形成和终止的因子的募集2,但该序列是如何启动的仍不清楚。在化学-遗传筛选中,人蛋白磷酸酶 1 (PP1) 亚型被鉴定为正转录延伸因子 b (P-TEFb) 的底物,也称为细胞周期蛋白依赖性激酶 9 (Cdk9)–细胞周期蛋白 T1 (CycT1) 复合物3 . 在这里,我们显示 Cdk9 和 PP1 控制裂殖酵母 Schizosaccharomyces pombe 中保守延伸因子 Spt5 的磷酸化。Cdk9 磷酸化 Spt5 和 PP1 亚型 Dis24 上的负调控位点。Spt5 羧基末端结构域中 Cdk9 靶向的位点可以在体外被 Dis2 去磷酸化,并且在体内抑制 Cdk9 后,dis2 突变会延迟 Spt5 去磷酸化。染色质免疫沉淀和测序分析表明,当转录复合物穿过裂解和聚腺苷酸化信号时,Spt5 被去磷酸化,伴随着 Pol II 在羧基末端结构域共有七肽重复序列 5 的残基 Ser2 处磷酸化的积累。条件致死的 Dis2 失活突变减弱了染色质上 Spt5 磷酸化的下降,促进超出正常终止区的转录(如通过精确连续转录和测序检测到的那样),并通过 Spt5 中 Cdk9 靶位点的消融进行遗传抑制。这些结部分通过控制延伸因子 Spt5 的磷酸化状态来控制裂殖酵母中的转录。
更新日期:2018-06-01
中文翻译:

Cdk9-PP1 开关调节 RNA 聚合酶 II 的延伸-终止转变
RNA 聚合酶 II (Pol II) 转录周期的结束受到严格监管,以防止相邻基因之间的干扰并保护转录组的完整性 1。Pol II 在切割和聚腺苷酸化信号下游的积累可以促进参与 mRNA 3'-末端形成和终止的因子的募集2,但该序列是如何启动的仍不清楚。在化学-遗传筛选中,人蛋白磷酸酶 1 (PP1) 亚型被鉴定为正转录延伸因子 b (P-TEFb) 的底物,也称为细胞周期蛋白依赖性激酶 9 (Cdk9)–细胞周期蛋白 T1 (CycT1) 复合物3 . 在这里,我们显示 Cdk9 和 PP1 控制裂殖酵母 Schizosaccharomyces pombe 中保守延伸因子 Spt5 的磷酸化。Cdk9 磷酸化 Spt5 和 PP1 亚型 Dis24 上的负调控位点。Spt5 羧基末端结构域中 Cdk9 靶向的位点可以在体外被 Dis2 去磷酸化,并且在体内抑制 Cdk9 后,dis2 突变会延迟 Spt5 去磷酸化。染色质免疫沉淀和测序分析表明,当转录复合物穿过裂解和聚腺苷酸化信号时,Spt5 被去磷酸化,伴随着 Pol II 在羧基末端结构域共有七肽重复序列 5 的残基 Ser2 处磷酸化的积累。条件致死的 Dis2 失活突变减弱了染色质上 Spt5 磷酸化的下降,促进超出正常终止区的转录(如通过精确连续转录和测序检测到的那样),并通过 Spt5 中 Cdk9 靶位点的消融进行遗传抑制。这些结部分通过控制延伸因子 Spt5 的磷酸化状态来控制裂殖酵母中的转录。











































 京公网安备 11010802027423号
京公网安备 11010802027423号