Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural basis of mitochondrial receptor binding and constriction by DRP1
Nature ( IF 50.5 ) Pub Date : 2018-06-01 , DOI: 10.1038/s41586-018-0211-2 Raghav Kalia , Ray Yu-Ruei Wang , Ali Yusuf , Paul V. Thomas , David A. Agard , Janet M. Shaw , Adam Frost
Nature ( IF 50.5 ) Pub Date : 2018-06-01 , DOI: 10.1038/s41586-018-0211-2 Raghav Kalia , Ray Yu-Ruei Wang , Ali Yusuf , Paul V. Thomas , David A. Agard , Janet M. Shaw , Adam Frost
|
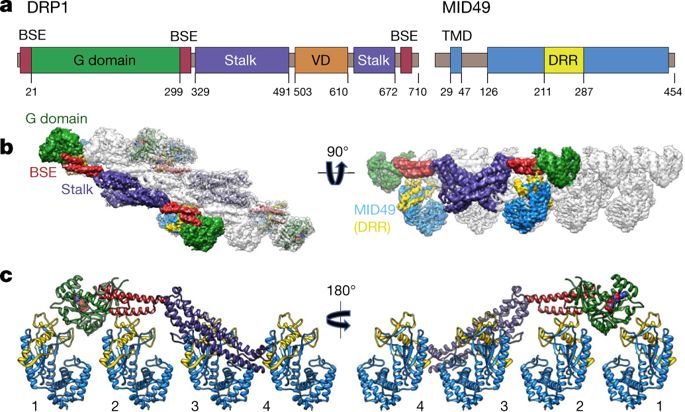
Mitochondrial inheritance, genome maintenance and metabolic adaptation depend on organelle fission by dynamin-related protein 1 (DRP1) and its mitochondrial receptors. DRP1 receptors include the paralogues mitochondrial dynamics proteins of 49 and 51 kDa (MID49 and MID51) and mitochondrial fission factor (MFF); however, the mechanisms by which these proteins recruit and regulate DRP1 are unknown. Here we present a cryo-electron microscopy structure of full-length human DRP1 co-assembled with MID49 and an analysis of structure- and disease-based mutations. We report that GTP induces a marked elongation and rotation of the GTPase domain, bundle-signalling element and connecting hinge loops of DRP1. In this conformation, a network of multivalent interactions promotes the polymerization of a linear DRP1 filament with MID49 or MID51. After co-assembly, GTP hydrolysis and exchange lead to MID receptor dissociation, filament shortening and curling of DRP1 oligomers into constricted and closed rings. Together, these views of full-length, receptor- and nucleotide-bound conformations reveal how DRP1 performs mechanical work through nucleotide-driven allostery.Cryo-electron microscopy is used to resolve the structure of human dynamin-related protein 1 co-assembled with its receptor mitochondrial dynamics protein of 49 kDa, along with an analysis of structure- and disease-based mutations.
中文翻译:

DRP1结合和收缩线粒体受体的结构基础
线粒体遗传、基因组维持和代谢适应取决于动力蛋白相关蛋白 1 (DRP1) 及其线粒体受体的细胞器裂变。DRP1受体包括49和51 kDa的旁系同源物线粒体动力学蛋白(MID49和MID51)和线粒体裂变因子(MFF);然而,这些蛋白质募集和调节 DRP1 的机制尚不清楚。在这里,我们展示了与 MID49 共同组装的全长人类 DRP1 的冷冻电子显微镜结构,以及基于结构和疾病的突变分析。我们报告 GTP 诱导 GTPase 域、束信号元件和 DRP1 的连接铰链环的显着伸长和旋转。在这种构象中,多价相互作用网络促进了线性 DRP1 细丝与 MID49 或 MID51 的聚合。共同组装后,GTP 水解和交换导致 MID 受体解离、细丝缩短和 DRP1 寡聚体卷曲成收缩和闭合的环。总之,这些全长、受体和核苷酸结合构象的观点揭示了 DRP1 如何通过核苷酸驱动的变构来执行机械工作。冷冻电子显微镜用于解析人类动力相关蛋白 1 与其共组装的结构49 kDa 的受体线粒体动力学蛋白,以及基于结构和疾病的突变分析。
更新日期:2018-06-01
中文翻译:

DRP1结合和收缩线粒体受体的结构基础
线粒体遗传、基因组维持和代谢适应取决于动力蛋白相关蛋白 1 (DRP1) 及其线粒体受体的细胞器裂变。DRP1受体包括49和51 kDa的旁系同源物线粒体动力学蛋白(MID49和MID51)和线粒体裂变因子(MFF);然而,这些蛋白质募集和调节 DRP1 的机制尚不清楚。在这里,我们展示了与 MID49 共同组装的全长人类 DRP1 的冷冻电子显微镜结构,以及基于结构和疾病的突变分析。我们报告 GTP 诱导 GTPase 域、束信号元件和 DRP1 的连接铰链环的显着伸长和旋转。在这种构象中,多价相互作用网络促进了线性 DRP1 细丝与 MID49 或 MID51 的聚合。共同组装后,GTP 水解和交换导致 MID 受体解离、细丝缩短和 DRP1 寡聚体卷曲成收缩和闭合的环。总之,这些全长、受体和核苷酸结合构象的观点揭示了 DRP1 如何通过核苷酸驱动的变构来执行机械工作。冷冻电子显微镜用于解析人类动力相关蛋白 1 与其共组装的结构49 kDa 的受体线粒体动力学蛋白,以及基于结构和疾病的突变分析。











































 京公网安备 11010802027423号
京公网安备 11010802027423号