Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Velocity-resolved kinetics of site-specific carbon monoxide oxidation on platinum surfaces
Nature ( IF 50.5 ) Pub Date : 2018-06-01 , DOI: 10.1038/s41586-018-0188-x Jannis Neugebohren , Dmitriy Borodin , Hinrich W. Hahn , Jan Altschäffel , Alexander Kandratsenka , Daniel J. Auerbach , Charles T. Campbell , Dirk Schwarzer , Dan J. Harding , Alec M. Wodtke , Theofanis N. Kitsopoulos
Nature ( IF 50.5 ) Pub Date : 2018-06-01 , DOI: 10.1038/s41586-018-0188-x Jannis Neugebohren , Dmitriy Borodin , Hinrich W. Hahn , Jan Altschäffel , Alexander Kandratsenka , Daniel J. Auerbach , Charles T. Campbell , Dirk Schwarzer , Dan J. Harding , Alec M. Wodtke , Theofanis N. Kitsopoulos
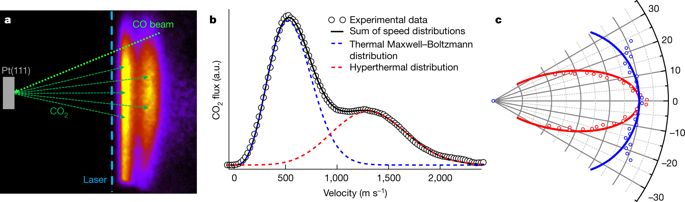
|
Catalysts are widely used to increase reaction rates. They function by stabilizing the transition state of the reaction at their active site, where the atomic arrangement ensures favourable interactions1. However, mechanistic understanding is often limited when catalysts possess multiple active sites—such as sites associated with either the step edges or the close-packed terraces of inorganic nanoparticles2–4—with distinct activities that cannot be measured simultaneously. An example is the oxidation of carbon monoxide over platinum surfaces, one of the oldest and best studied heterogeneous reactions. In 1824, this reaction was recognized to be crucial for the function of the Davy safety lamp, and today it is used to optimize combustion, hydrogen production and fuel-cell operation5,6. The carbon dioxide products are formed in a bimodal kinetic energy distribution7–13; however, despite extensive study5, it remains unclear whether this reflects the involvement of more than one reaction mechanism occurring at multiple active sites12,13. Here we show that the reaction rates at different active sites can be measured simultaneously, using molecular beams to controllably introduce reactants and slice ion imaging14,15 to map the velocity vectors of the product molecules, which reflect the symmetry and the orientation of the active site16. We use this velocity-resolved kinetics approach to map the oxidation rates of carbon monoxide at step edges and terrace sites on platinum surfaces, and find that the reaction proceeds through two distinct channels11–13: it is dominated at low temperatures by the more active step sites, and at high temperatures by the more abundant terrace sites. We expect our approach to be applicable to a wide range of heterogeneous reactions and to provide improved mechanistic understanding of the contribution of different active sites, which should be useful in the design of improved catalysts.The catalytic oxidation of carbon monoxide over platinum proceeds through two distinct channels: it is dominated at low temperatures by the more active step sites and at high temperatures by the more abundant terrace sites of the platinum surface.
中文翻译:

铂表面特定位点一氧化碳氧化的速度分辨动力学
催化剂被广泛用于提高反应速率。它们通过在其活性位点稳定反应的过渡态来发挥作用,其中原子排列确保了有利的相互作用 1。然而,当催化剂具有多个活性位点(例如与阶梯边缘或无机纳米粒子 2-4 的密堆积平台相关的位点)时,机理理解通常是有限的,这些活性位点不能同时测量。一个例子是一氧化碳在铂表面的氧化,这是最古老和研究最多的多相反应之一。1824 年,人们认识到这种反应对戴维安全灯的功能至关重要,如今它被用于优化燃烧、制氢和燃料电池运行 5,6。二氧化碳产物以双峰动能分布 7-13 形成;然而,尽管进行了广泛的研究 5,但仍不清楚这是否反映了在多个活性位点发生的不止一种反应机制的参与 12、13。在这里,我们展示了可以同时测量不同活性位点的反应速率,使用分子束可控地引入反应物和切片离子成像 14,15 来映射产物分子的速度矢量,这反映了活性位点的对称性和方向 16 . 我们使用这种速度分辨动力学方法来绘制一氧化碳在铂表面台阶边缘和平台位点的氧化速率,并发现反应通过两个不同的通道进行 11-13:它在低温下由更活跃的步骤支配网站,并且在更丰富的梯田地点的高温下。我们希望我们的方法适用于广泛的非均相反应,并提供对不同活性位点贡献的更好的机理理解,这应该有助于设计改进的催化剂。一氧化碳在铂上的催化氧化通过两个过程进行不同的通道:它在低温下由更活跃的台阶位点控制,在高温下由铂表面的更丰富的平台位点控制。
更新日期:2018-06-01
中文翻译:

铂表面特定位点一氧化碳氧化的速度分辨动力学
催化剂被广泛用于提高反应速率。它们通过在其活性位点稳定反应的过渡态来发挥作用,其中原子排列确保了有利的相互作用 1。然而,当催化剂具有多个活性位点(例如与阶梯边缘或无机纳米粒子 2-4 的密堆积平台相关的位点)时,机理理解通常是有限的,这些活性位点不能同时测量。一个例子是一氧化碳在铂表面的氧化,这是最古老和研究最多的多相反应之一。1824 年,人们认识到这种反应对戴维安全灯的功能至关重要,如今它被用于优化燃烧、制氢和燃料电池运行 5,6。二氧化碳产物以双峰动能分布 7-13 形成;然而,尽管进行了广泛的研究 5,但仍不清楚这是否反映了在多个活性位点发生的不止一种反应机制的参与 12、13。在这里,我们展示了可以同时测量不同活性位点的反应速率,使用分子束可控地引入反应物和切片离子成像 14,15 来映射产物分子的速度矢量,这反映了活性位点的对称性和方向 16 . 我们使用这种速度分辨动力学方法来绘制一氧化碳在铂表面台阶边缘和平台位点的氧化速率,并发现反应通过两个不同的通道进行 11-13:它在低温下由更活跃的步骤支配网站,并且在更丰富的梯田地点的高温下。我们希望我们的方法适用于广泛的非均相反应,并提供对不同活性位点贡献的更好的机理理解,这应该有助于设计改进的催化剂。一氧化碳在铂上的催化氧化通过两个过程进行不同的通道:它在低温下由更活跃的台阶位点控制,在高温下由铂表面的更丰富的平台位点控制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号