当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Ter-Ionic Complex that Forms a Bond Upon Visible Light Absorption
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-06-13 , DOI: 10.1021/jacs.8b04961 Sara A. M. Wehlin 1 , Ludovic Troian-Gautier 1 , Renato N. Sampaio 1 , Lionel Marcélis 2 , Gerald J. Meyer 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-06-13 , DOI: 10.1021/jacs.8b04961 Sara A. M. Wehlin 1 , Ludovic Troian-Gautier 1 , Renato N. Sampaio 1 , Lionel Marcélis 2 , Gerald J. Meyer 1
Affiliation
|
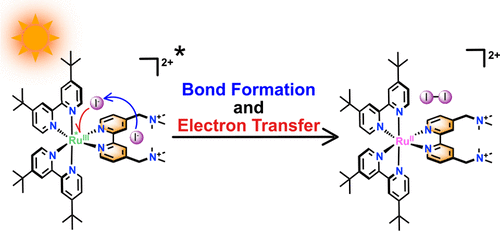
A "ter-ionic complex" composed of a tetracationic Ru(II) complex and two iodide ions was found to yield a covalent I-I bond upon visible light excitation in acetone solution. 1H NMR, visible absorption and DFT studies revealed that one iodide was associated with a ligand while the other was closer to the Ru metal center. Standard Stern-Volmer quenching of the excited state by iodide revealed upward curvature with a novel saturation at high concentrations. The data were fully consistent with a mechanism in which the Ru metal center in the excited state accepts an electron from iodide to form an iodine atom and, within 70 ns, that atom reacts with the iodide associated with the ligand to yield I2•-. This rapid formation of an I-I bond was facilitated by the supramolecular assembly of the three reactant ions necessary for this ter-ionic reaction that is relevant to solar fuel production.
中文翻译:

在可见光吸收时形成键的三离子复合物
发现由四阳离子 Ru(II) 络合物和两个碘离子组成的“三离子络合物”在丙酮溶液中的可见光激发下产生共价 II 键。1H NMR、可见光吸收和 DFT 研究表明,一种碘化物与配体结合,而另一种更接近 Ru 金属中心。碘化物对激发态的标准 Stern-Volmer 淬灭揭示了在高浓度下具有新饱和度的向上弯曲。数据与处于激发态的 Ru 金属中心接受来自碘化物的电子形成碘原子的机制完全一致,并且在 70 ns 内,该原子与与配体相关的碘化物反应生成 I2•-。
更新日期:2018-06-13
中文翻译:

在可见光吸收时形成键的三离子复合物
发现由四阳离子 Ru(II) 络合物和两个碘离子组成的“三离子络合物”在丙酮溶液中的可见光激发下产生共价 II 键。1H NMR、可见光吸收和 DFT 研究表明,一种碘化物与配体结合,而另一种更接近 Ru 金属中心。碘化物对激发态的标准 Stern-Volmer 淬灭揭示了在高浓度下具有新饱和度的向上弯曲。数据与处于激发态的 Ru 金属中心接受来自碘化物的电子形成碘原子的机制完全一致,并且在 70 ns 内,该原子与与配体相关的碘化物反应生成 I2•-。











































 京公网安备 11010802027423号
京公网安备 11010802027423号