当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Search of Selectivity in Inhibition of ADAM10
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2018-06-11 00:00:00 , DOI: 10.1021/acsmedchemlett.8b00163 Kiran V. Mahasenan 1 , Derong Ding 1 , Ming Gao 1 , Trung T. Nguyen 1 , Mark A. Suckow 2 , Valerie A. Schroeder 2 , William R. Wolter 2 , Mayland Chang 1 , Shahriar Mobashery 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2018-06-11 00:00:00 , DOI: 10.1021/acsmedchemlett.8b00163 Kiran V. Mahasenan 1 , Derong Ding 1 , Ming Gao 1 , Trung T. Nguyen 1 , Mark A. Suckow 2 , Valerie A. Schroeder 2 , William R. Wolter 2 , Mayland Chang 1 , Shahriar Mobashery 1
Affiliation
|
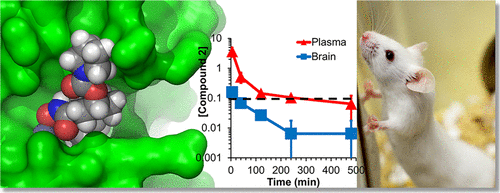
The metalloproteinase ADAM10 has been reported as an important target for drug discovery in several human diseases. In this vein, (6S,7S)-N-hydroxy-5-methyl-6-(4-(5-(trifluoromethyl)pyridin-2-yl)piperazine-1-carbonyl)-5-azaspiro[2.5]octane-7-carboxamide (compound 1) has been reported as a selective ADAM10 inhibitor. We synthesized this compound and document that it lacks both potency and selectivity in inhibition of ADAM10. This finding necessitated a structure-based computational analysis to investigate potency and selectivity of ADAM10 inhibition. The model that emerged indeed excluded compound 1 as an inhibitor for ADAM10, while suggesting another reported compound, (1R,3S,4S)-3-(hydroxycarbamoyl)-4-(4-phenylpiperidine-1-carbonyl)cyclohexyl pyrrolidine-1-carboxylate (compound 2), as an ADAM10 selective inhibitor. Compound 2 was synthesized and its potency, and selectivity in inhibition of ADAM10 were documented with a panel of several related enzymes. Pharmacokinetic studies of compound 2 in mice documented that the compound crosses the blood–brain barrier and may be useful as a pharmacological agent or mechanistic tool to delineate the role of ADAM10 in neurological diseases.
中文翻译:

寻找选择性抑制ADAM10
据报道,金属蛋白酶ADAM10是在几种人类疾病中发现药物的重要靶标。在这种情况下,(6 S,7 S)-N-羟基-5-甲基-6-(4-(5-(三氟甲基)吡啶-2-基)哌嗪-1-羰基)-5-氮杂螺[2.5]据报道,辛烷-7-羧酰胺(化合物1)是一种选择性ADAM10抑制剂。我们合成了该化合物,并证明了它在抑制ADAM10方面既缺乏效力,又缺乏选择性。这一发现有必要进行基于结构的计算分析,以研究ADAM10抑制的效力和选择性。出现的模型确实排除了化合物1作为ADAM10的抑制剂,同时暗示了另一种报道的化合物,(1 R,3S,4S)-3-(羟基氨基甲酰基)-4-(4-苯基哌啶-1-羰基)环己基吡咯烷-1-羧酸酯(化合物2),作为ADAM10选择性抑制剂。合成了化合物2,并用一组相关酶记录了其效力和抑制ADAM10的选择性。化合物2在小鼠中的药代动力学研究表明,该化合物可穿越血脑屏障,可作为药理剂或机制工具来描述ADAM10在神经系统疾病中的作用。
更新日期:2018-06-11
中文翻译:

寻找选择性抑制ADAM10
据报道,金属蛋白酶ADAM10是在几种人类疾病中发现药物的重要靶标。在这种情况下,(6 S,7 S)-N-羟基-5-甲基-6-(4-(5-(三氟甲基)吡啶-2-基)哌嗪-1-羰基)-5-氮杂螺[2.5]据报道,辛烷-7-羧酰胺(化合物1)是一种选择性ADAM10抑制剂。我们合成了该化合物,并证明了它在抑制ADAM10方面既缺乏效力,又缺乏选择性。这一发现有必要进行基于结构的计算分析,以研究ADAM10抑制的效力和选择性。出现的模型确实排除了化合物1作为ADAM10的抑制剂,同时暗示了另一种报道的化合物,(1 R,3S,4S)-3-(羟基氨基甲酰基)-4-(4-苯基哌啶-1-羰基)环己基吡咯烷-1-羧酸酯(化合物2),作为ADAM10选择性抑制剂。合成了化合物2,并用一组相关酶记录了其效力和抑制ADAM10的选择性。化合物2在小鼠中的药代动力学研究表明,该化合物可穿越血脑屏障,可作为药理剂或机制工具来描述ADAM10在神经系统疾病中的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号