当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal Binding to Amyloid-β1-42: A Ligand Field Molecular Dynamics Study.
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-06-28 , DOI: 10.1021/acschemneuro.8b00210 Shaun T Mutter 1 , Matthew Turner 1 , Robert J Deeth 2 , James A Platts 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-06-28 , DOI: 10.1021/acschemneuro.8b00210 Shaun T Mutter 1 , Matthew Turner 1 , Robert J Deeth 2 , James A Platts 1
Affiliation
|
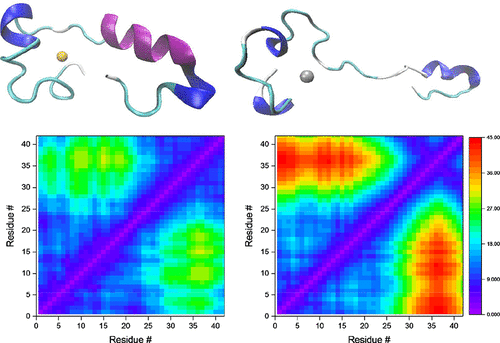
Ligand field molecular mechanics simulation has been used to model the interactions of copper(II) and platinum(II) with the amyloid-β1-42 peptide monomer. Molecular dynamics over several microseconds for both metalated systems are compared to analogous results for the free peptide. Significant differences in structural parameters are observed, both between Cu and Pt bound systems as well as between free and metal-bound peptide. Both metals stabilize the formation of helices in the peptide as well as reducing the content of β secondary structural elements compared to the unbound monomer. This is in agreement with experimental reports of metals reducing β-sheet structures, leading to formation of amorphous aggregates over amyloid fibrils. The shape and size of the peptide structures also undergo noteworthy change, with the free peptide exhibiting globular-like structure, platinum(II) system adopting extended structures, and copper(II) system resulting in a mixture of conformations similar to both of these. Salt bridge networks exhibit major differences: the Asp23-Lys28 salt bridge, known to be important in fibril formation, has a differing distance profile within all three systems studied. Salt bridges in the metal binding region of the peptide are strongly altered; in particular, the Arg5-Asp7 salt bridge, which has an occurrence of 71% in the free peptide, is reduced to zero in the presence of both metals.
中文翻译:

金属与淀粉样蛋白β1-42的结合:配体场分子动力学研究。
配体场分子力学模拟已用于模拟铜(II)和铂(II)与淀粉样β1-42肽单体的相互作用。将两种金属化系统在几微秒内的分子动力学与游离肽的类似结果进行比较。在Cu和Pt结合的系统之间以及在游离和金属结合的肽之间都观察到结构参数的显着差异。与未结合的单体相比,两种金属均能稳定肽中螺旋的形成并降低β二级结构元素的含量。这与金属还原β-折叠结构,导致淀粉样蛋白原纤维上形成无定形聚集体的实验报告一致。肽结构的形状和大小也发生了显着变化,游离肽呈球状结构,铂(II)体系采用延伸结构,铜(II)体系产生类似于这两种构象的混合物。盐桥网络表现出主要差异:已知在原纤维形成中很重要的Asp23-Lys28盐桥在所有研究的三个系统中的距离分布都不同。肽的金属结合区中的盐桥被强烈改变;特别地,在两种金属的存在下,在游离肽中存在71%的Arg5-Asp7盐桥被还原为零。已知对原纤维形成很重要,在所有研究的三个系统中距离分布都不同。肽的金属结合区中的盐桥被强烈改变;特别地,在两种金属的存在下,在游离肽中存在71%的Arg5-Asp7盐桥被还原为零。已知对原纤维形成很重要,在所有研究的三个系统中距离分布都不同。肽的金属结合区中的盐桥被强烈改变;尤其是,在两种金属的存在下,在游离肽中有71%的Arg5-Asp7盐桥被还原为零。
更新日期:2018-06-13
中文翻译:

金属与淀粉样蛋白β1-42的结合:配体场分子动力学研究。
配体场分子力学模拟已用于模拟铜(II)和铂(II)与淀粉样β1-42肽单体的相互作用。将两种金属化系统在几微秒内的分子动力学与游离肽的类似结果进行比较。在Cu和Pt结合的系统之间以及在游离和金属结合的肽之间都观察到结构参数的显着差异。与未结合的单体相比,两种金属均能稳定肽中螺旋的形成并降低β二级结构元素的含量。这与金属还原β-折叠结构,导致淀粉样蛋白原纤维上形成无定形聚集体的实验报告一致。肽结构的形状和大小也发生了显着变化,游离肽呈球状结构,铂(II)体系采用延伸结构,铜(II)体系产生类似于这两种构象的混合物。盐桥网络表现出主要差异:已知在原纤维形成中很重要的Asp23-Lys28盐桥在所有研究的三个系统中的距离分布都不同。肽的金属结合区中的盐桥被强烈改变;特别地,在两种金属的存在下,在游离肽中存在71%的Arg5-Asp7盐桥被还原为零。已知对原纤维形成很重要,在所有研究的三个系统中距离分布都不同。肽的金属结合区中的盐桥被强烈改变;特别地,在两种金属的存在下,在游离肽中存在71%的Arg5-Asp7盐桥被还原为零。已知对原纤维形成很重要,在所有研究的三个系统中距离分布都不同。肽的金属结合区中的盐桥被强烈改变;尤其是,在两种金属的存在下,在游离肽中有71%的Arg5-Asp7盐桥被还原为零。











































 京公网安备 11010802027423号
京公网安备 11010802027423号