当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Aminocaprolactam Racemase from Ochrobactrum anthropi with Promiscuous Amino Acid Ester Racemase Activity
ChemBioChem ( IF 2.6 ) Pub Date : 2018-07-10 , DOI: 10.1002/cbic.201800265 Amina Frese 1 , Sarah V Barrass 1 , Peter W Sutton 2, 3 , Joe P Adams 2 , Gideon Grogan 1
ChemBioChem ( IF 2.6 ) Pub Date : 2018-07-10 , DOI: 10.1002/cbic.201800265 Amina Frese 1 , Sarah V Barrass 1 , Peter W Sutton 2, 3 , Joe P Adams 2 , Gideon Grogan 1
Affiliation
|
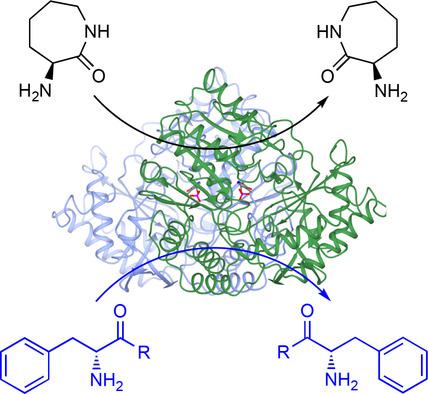
Kinetic resolution: α‐Amino ϵ‐caprolactam racemase (ACLR) from O. anthropi catalyses the racemisation of ϵ‐ACL, but also of phenylalanine amide and phenylalanine methyl ester, the latter an unprecedented substrate for ACLRs. We have determined the structure of OaACLR and used this to engineer a mutant with a 3.7‐fold greater activity than the wild type for the amino acid ester racemisation.

中文翻译:

具有混杂氨基酸酯消旋酶活性的来自人苍白杆菌的氨基己内酰胺消旋酶
动力学解析:来自O. anthropi的 α-氨基 ε-己内酰胺消旋酶 (ACLR) 催化 ε-ACL 的外消旋化,同时也催化苯丙氨酸酰胺和苯丙氨酸甲酯的外消旋化,后者是 ACLR 前所未有的底物。我们已经确定了Oa ACLR 的结构,并用它设计了一个突变体,其氨基酸酯外消旋活性比野生型高 3.7 倍。

更新日期:2018-07-10

中文翻译:

具有混杂氨基酸酯消旋酶活性的来自人苍白杆菌的氨基己内酰胺消旋酶
动力学解析:来自O. anthropi的 α-氨基 ε-己内酰胺消旋酶 (ACLR) 催化 ε-ACL 的外消旋化,同时也催化苯丙氨酸酰胺和苯丙氨酸甲酯的外消旋化,后者是 ACLR 前所未有的底物。我们已经确定了Oa ACLR 的结构,并用它设计了一个突变体,其氨基酸酯外消旋活性比野生型高 3.7 倍。












































 京公网安备 11010802027423号
京公网安备 11010802027423号