当前位置:
X-MOL 学术
›
Biomaterials
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Perivascular signals alter global gene expression profile of glioblastoma and response to temozolomide in a gelatin hydrogel.
Biomaterials ( IF 12.8 ) Pub Date : 2018-06-13 , DOI: 10.1016/j.biomaterials.2018.06.013 Mai T Ngo 1 , Brendan A C Harley 2
Biomaterials ( IF 12.8 ) Pub Date : 2018-06-13 , DOI: 10.1016/j.biomaterials.2018.06.013 Mai T Ngo 1 , Brendan A C Harley 2
Affiliation
|
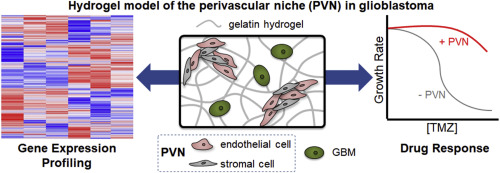
Glioblastoma (GBM) is the most common primary malignant brain tumor, with patients exhibiting poor survival (median survival time: 15 months). Difficulties in treating GBM include not only the inability to resect the diffusively-invading tumor cells, but also therapeutic resistance. The perivascular niche (PVN) within the GBM tumor microenvironment contributes significantly to tumor cell invasion, cancer stem cell maintenance, and has been shown to protect tumor cells from radiation and chemotherapy. In this study, we examine how the inclusion of non-tumor cells in culture with tumor cells within a hydrogel impacts the overall gene expression profile of an in vitro artificial perivascular niche (PVN) comprised of endothelial and stromal cells directly cultured with GBM tumor cells within a methacrylamide-functionalized gelatin hydrogel. Using RNA-seq, we demonstrate that genes related to angiogenesis and extracellular matrix remodeling are upregulated in the PVN model compared to hydrogels containing only tumor or perivascular niche cells, while downregulated genes are related to cell cycle and DNA damage repair. Signaling pathways and genes commonly implicated in GBM malignancy, such as MGMT, EGFR, PI3K-Akt signaling, and Ras/MAPK signaling are also upregulated in the PVN model. We describe the kinetics of gene expression within the PVN hydrogels over a course of 14 days, observing the patterns associated with tumor cell-mediated endothelial network co-option and regression. We finally examine the effect of temozolomide, a frontline chemotherapy used clinically against GBM, on the PVN culture. Notably, the PVN model is less responsive to TMZ compared to hydrogels containing only tumor cells. Overall, these results demonstrate that inclusion of cellular and matrix-associated elements of the PVN within an in vitro model of GBM allows for the development of gene expression patterns and therapeutic response relevant to GBM.
中文翻译:

周血管信号改变胶质母细胞瘤的整体基因表达谱和明胶水凝胶中对替莫唑胺的反应。
胶质母细胞瘤(GBM)是最常见的原发性恶性脑肿瘤,患者生存期较差(中位生存时间:15个月)。治疗GBM的困难不仅包括不能切除扩散侵袭的肿瘤细胞,还包括治疗抗性。GBM肿瘤微环境中的血管周围小生境(PVN)对肿瘤细胞侵袭,癌症干细胞维持有重要贡献,并已显示出保护肿瘤细胞免于放射和化学疗法的作用。在这项研究中,我们研究了在水凝胶中与肿瘤细胞一起培养的非肿瘤细胞的包容如何影响体外人工血管周生境(PVN)的整体基因表达谱,该环境由直接与GBM肿瘤细胞培养的内皮细胞和基质细胞组成在甲基丙烯酰胺官能化的明胶水凝胶中。使用RNA序列,我们证明与仅含有肿瘤或血管周围小生境细胞的水凝胶相比,PVN模型中与血管生成和细胞外基质重塑有关的基因被上调,而被下调的基因则与细胞周期和DNA损伤修复有关。在PVN模型中,通常与GBM恶性肿瘤有关的信号传导途径和基因(例如MGMT,EGFR,PI3K-Akt信号传导和Ras / MAPK信号传导)也被上调。我们描述了在14天的过程中PVN水凝胶内基因表达的动力学,观察了与肿瘤细胞介导的内皮网络共同选择和回归相关的模式。最后,我们检查了替莫唑胺(一种临床上用于对抗GBM的一线化疗药物)对PVN培养的影响。尤其,与仅包含肿瘤细胞的水凝胶相比,PVN模型对TMZ的反应较弱。总体而言,这些结果表明,在GBM的体外模型中纳入PVN的细胞和基质相关元素,可以促进与GBM相关的基因表达模式和治疗反应的发展。
更新日期:2018-11-29
中文翻译:

周血管信号改变胶质母细胞瘤的整体基因表达谱和明胶水凝胶中对替莫唑胺的反应。
胶质母细胞瘤(GBM)是最常见的原发性恶性脑肿瘤,患者生存期较差(中位生存时间:15个月)。治疗GBM的困难不仅包括不能切除扩散侵袭的肿瘤细胞,还包括治疗抗性。GBM肿瘤微环境中的血管周围小生境(PVN)对肿瘤细胞侵袭,癌症干细胞维持有重要贡献,并已显示出保护肿瘤细胞免于放射和化学疗法的作用。在这项研究中,我们研究了在水凝胶中与肿瘤细胞一起培养的非肿瘤细胞的包容如何影响体外人工血管周生境(PVN)的整体基因表达谱,该环境由直接与GBM肿瘤细胞培养的内皮细胞和基质细胞组成在甲基丙烯酰胺官能化的明胶水凝胶中。使用RNA序列,我们证明与仅含有肿瘤或血管周围小生境细胞的水凝胶相比,PVN模型中与血管生成和细胞外基质重塑有关的基因被上调,而被下调的基因则与细胞周期和DNA损伤修复有关。在PVN模型中,通常与GBM恶性肿瘤有关的信号传导途径和基因(例如MGMT,EGFR,PI3K-Akt信号传导和Ras / MAPK信号传导)也被上调。我们描述了在14天的过程中PVN水凝胶内基因表达的动力学,观察了与肿瘤细胞介导的内皮网络共同选择和回归相关的模式。最后,我们检查了替莫唑胺(一种临床上用于对抗GBM的一线化疗药物)对PVN培养的影响。尤其,与仅包含肿瘤细胞的水凝胶相比,PVN模型对TMZ的反应较弱。总体而言,这些结果表明,在GBM的体外模型中纳入PVN的细胞和基质相关元素,可以促进与GBM相关的基因表达模式和治疗反应的发展。









































 京公网安备 11010802027423号
京公网安备 11010802027423号