Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Study of Near-Band-Edge Property in Oxygen-Incorporated ZnS for Acting as an Efficient Crystal Photocatalyst.
ACS Omega ( IF 3.7 ) Pub Date : 2018-06-13 , DOI: 10.1021/acsomega.8b00260 Min-Han Lin,Perumalswamy Sekar Parasuraman,Ching-Hwa Ho
ACS Omega ( IF 3.7 ) Pub Date : 2018-06-13 , DOI: 10.1021/acsomega.8b00260 Min-Han Lin,Perumalswamy Sekar Parasuraman,Ching-Hwa Ho
|
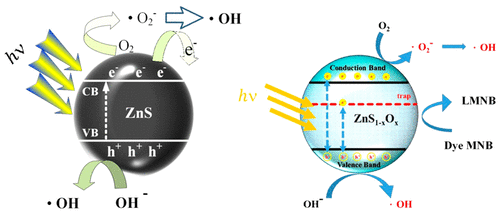
A wide gap semiconductor material has attracted attention as a heterophotocatalyst because of its light harvesting nature to be used in alternative energy production for the next generation. We, herein, grow and synthesize ZnS(1-x)O x series compounds using the chemical vapor transport (CVT) method with I2 serving as the transport agent. Different crystals, such as undoped ZnS and oxygen-doped ZnS0.94O0.06 and ZnS0.88O0.12, revealed different bright palette emissions that were presented in photoluminescence spectra in our previous report. To study the electron-hole pair interaction of this sample series, the near-band-edge transitions of the sample series were characterized in detail by photoconductivity (PC) experiments. Additional results from surface photovoltage (SPV) spectra also detected the surface and defect-edge transitions from the higher oxygen-doped ZnS crystals. PC measurement results showed a red-shift in the bandgap with increasing incorporation of oxygen on ZnS. Consequently, the samples were subjected to photoirradiation by xenon lamp for the degradation of methylene blue (MNB) by acting as heterophotocatalysts. Undoped ZnS emerged as the best photocatalyst candidate with the fastest rate constant value of 0.0277 min-1. In cubic {111} ZnS [{111} c-ZnS], the polarized Zn+ → S- ions may play a vital role as a photocatalyst because of their strong electron-hole polarization, which leads to the mechanism for degradation of the MNB solution.
中文翻译:

掺入氧的ZnS的近谱带特性研究作为高效晶体光催化剂。
宽间隙半导体材料作为一种异质光催化剂已经吸引了人们的注意,因为它具有光收集的特性,可用于下一代的替代能源生产中。我们在这里使用I2作为传输剂,使用化学气相传输(CVT)方法生长和合成ZnS(1-x)O x系列化合物。不同的晶体,例如未掺杂的ZnS和氧掺杂的ZnS0.94O0.06和ZnS0.88O0.12,揭示了不同的亮调色板发射,这些发光在我们先前的报告中显示在光致发光光谱中。为了研究该样品系列的电子-空穴对相互作用,通过光电导(PC)实验详细描述了该样品系列的近带边跃迁。表面光电压(SPV)光谱的其他结果还检测到较高掺杂氧的ZnS晶体的表面和缺陷边缘跃迁。PC测量结果显示,随着氧在ZnS上的掺入增加,带隙发生红移。因此,样品通过用作氙气灯作为杂光催化剂而经受氙灯的光辐照,以降解亚甲基蓝(MNB)。未掺杂的ZnS以0.0277 min-1的最快速率常数值出现,成为最佳的光催化剂候选者。在立方{111} ZnS [{111} c-ZnS]中,极化的Zn +→S-离子可能由于其强大的电子-空穴极化作用而起着光催化剂的重要作用,这导致了MNB溶液的降解机理。PC测量结果显示,随着氧在ZnS上的掺入增加,带隙发生红移。因此,样品通过用作氙气灯作为杂光催化剂而经受氙灯的光辐照,以降解亚甲基蓝(MNB)。未掺杂的ZnS以0.0277 min-1的最快速率常数值出现,成为最佳的光催化剂候选者。在立方{111} ZnS [{111} c-ZnS]中,极化的Zn +→S-离子可能由于其强大的电子-空穴极化作用而起着光催化剂的重要作用,这导致了MNB溶液的降解机理。PC测量结果显示,随着氧在ZnS上的掺入增加,带隙发生红移。因此,通过用作杂光催化剂,用氙气灯对样品进行亚甲基蓝(MNB)的降解。未掺杂的ZnS以0.0277 min-1的最快速率常数值出现,成为最佳的光催化剂候选者。在立方{111} ZnS [{111} c-ZnS]中,极化的Zn +→S-离子可能由于其强大的电子-空穴极化作用而起着光催化剂的重要作用,这导致了MNB溶液的降解机理。未掺杂的ZnS以0.0277 min-1的最快速率常数值出现,成为最佳的光催化剂候选者。在立方{111} ZnS [{111} c-ZnS]中,极化的Zn +→S-离子可能由于其强大的电子-空穴极化作用而起着光催化剂的重要作用,这导致了MNB溶液的降解机理。未掺杂的ZnS以0.0277 min-1的最快速率常数值出现,成为最佳的光催化剂候选者。在立方{111} ZnS [{111} c-ZnS]中,极化的Zn +→S-离子可能由于其强大的电子-空穴极化作用而起着光催化剂的重要作用,这导致了MNB溶液的降解机理。
更新日期:2018-06-13
中文翻译:

掺入氧的ZnS的近谱带特性研究作为高效晶体光催化剂。
宽间隙半导体材料作为一种异质光催化剂已经吸引了人们的注意,因为它具有光收集的特性,可用于下一代的替代能源生产中。我们在这里使用I2作为传输剂,使用化学气相传输(CVT)方法生长和合成ZnS(1-x)O x系列化合物。不同的晶体,例如未掺杂的ZnS和氧掺杂的ZnS0.94O0.06和ZnS0.88O0.12,揭示了不同的亮调色板发射,这些发光在我们先前的报告中显示在光致发光光谱中。为了研究该样品系列的电子-空穴对相互作用,通过光电导(PC)实验详细描述了该样品系列的近带边跃迁。表面光电压(SPV)光谱的其他结果还检测到较高掺杂氧的ZnS晶体的表面和缺陷边缘跃迁。PC测量结果显示,随着氧在ZnS上的掺入增加,带隙发生红移。因此,样品通过用作氙气灯作为杂光催化剂而经受氙灯的光辐照,以降解亚甲基蓝(MNB)。未掺杂的ZnS以0.0277 min-1的最快速率常数值出现,成为最佳的光催化剂候选者。在立方{111} ZnS [{111} c-ZnS]中,极化的Zn +→S-离子可能由于其强大的电子-空穴极化作用而起着光催化剂的重要作用,这导致了MNB溶液的降解机理。PC测量结果显示,随着氧在ZnS上的掺入增加,带隙发生红移。因此,样品通过用作氙气灯作为杂光催化剂而经受氙灯的光辐照,以降解亚甲基蓝(MNB)。未掺杂的ZnS以0.0277 min-1的最快速率常数值出现,成为最佳的光催化剂候选者。在立方{111} ZnS [{111} c-ZnS]中,极化的Zn +→S-离子可能由于其强大的电子-空穴极化作用而起着光催化剂的重要作用,这导致了MNB溶液的降解机理。PC测量结果显示,随着氧在ZnS上的掺入增加,带隙发生红移。因此,通过用作杂光催化剂,用氙气灯对样品进行亚甲基蓝(MNB)的降解。未掺杂的ZnS以0.0277 min-1的最快速率常数值出现,成为最佳的光催化剂候选者。在立方{111} ZnS [{111} c-ZnS]中,极化的Zn +→S-离子可能由于其强大的电子-空穴极化作用而起着光催化剂的重要作用,这导致了MNB溶液的降解机理。未掺杂的ZnS以0.0277 min-1的最快速率常数值出现,成为最佳的光催化剂候选者。在立方{111} ZnS [{111} c-ZnS]中,极化的Zn +→S-离子可能由于其强大的电子-空穴极化作用而起着光催化剂的重要作用,这导致了MNB溶液的降解机理。未掺杂的ZnS以0.0277 min-1的最快速率常数值出现,成为最佳的光催化剂候选者。在立方{111} ZnS [{111} c-ZnS]中,极化的Zn +→S-离子可能由于其强大的电子-空穴极化作用而起着光催化剂的重要作用,这导致了MNB溶液的降解机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号