当前位置:
X-MOL 学术
›
Photochem. Photobiol. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photochemistry of 9-acridinecarboxaldehyde in aqueous media†
Photochemical & Photobiological Sciences ( IF 2.7 ) Pub Date : 2018-06-12 00:00:00 , DOI: 10.1039/c8pp00185e Wolf-Ulrich Palm 1, 2, 3, 4
Photochemical & Photobiological Sciences ( IF 2.7 ) Pub Date : 2018-06-12 00:00:00 , DOI: 10.1039/c8pp00185e Wolf-Ulrich Palm 1, 2, 3, 4
Affiliation
|
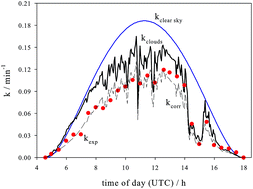
Dark and photolysis reactions in solution were investigated for 9-acridinecarboxaldehyde (ACL). ACL reacts in the dark at T = 20 °C and pH = 7.0 in an air saturated solution to the main product 9-acridinecarboxylic acid (ACA) and to the minor product 9-acridinemethanol (ACM) with a lifetime of τ = 4.3 days. The dissociation constant of the base ACLH+ was determined to be pKa ± σ = 4.38 ± 0.04. The photolysis of ACL was investigated using a polychromatic Xe-light source. The quantum yield in aqueous solution at T = 20 °C in a concentration range of c0(ACL) = 0.18–16.6 μM for pH > pKa and for nitrogen, air and oxygen aerated solutions was found to be Φ ± σ = (0.015 ± 0.003) mol/mol, independent from concentration. The quantum yield of ACLH+, i.e. for pH ≪ pKa, is by a factor of 2 higher (Φ = 0.029 mol/mol). Quantum yields in methanol and isopropanol are slightly lower compared to water and in acetone lower by about a factor of 20. In acetonitrile ACL was found to be practically photostable. Minimum lifetimes in sunlight for a measurement on September 5, 2017 were in the range of τ = 5–10 minutes. The diurnal photolysis of ACL in sunlight was satisfactory explained using the mean quantum yield, the absorption spectrum and photon fluxes with suitable corrections for cloudiness and the dimensions of the setup. For low concentrations ACR is formed with a yield of practically 100% in the photolysis reaction. However, with increasing concentration of ACL yields of ACR decrease and yields of ACA increase. 9(10H)-Acridinone and ACM were always detected as minor products with yields below 2%. 9-Methylacridine was never detected in any reaction of ACL. Strong indications are presented of a photolysis reaction of ACL in a river located in Lower Saxony (Germany) with a corresponding equimolar formation of ACR. ACL is therefore a direct precursor of ACR in natural surface water.
中文翻译:

水性介质中9-ac啶甲醛的光化学作用†
研究了溶液中的9-ac啶甲醛(ACL)的黑暗反应和光解反应。ACL在空气饱和溶液中,在T = 20°C和pH = 7.0的黑暗中与主要产物9-ac啶甲酸(ACA)和次要产物9-ac啶甲醇(ACM)反应,寿命τ = 4.3天。确定基本ACLH +的解离常数为p K a ± σ = 4.38±0.04。使用多色Xe光源研究了ACL的光解作用。pH> p K a时,在T = 20°C的水溶液中,在c 0(ACL)= 0.18–16.6μM的浓度范围内,量子产率对于氮气,空气和氧气充气溶液的浓度为Φ ± σ =(0.015±0.003)mol / mol。ACLH +的量子产率,即pH≪ p K a的量子产率高出2倍(Φ = 0.029 mol / mol)。与水相比,甲醇和异丙醇中的量子产率略低,而丙酮中的量子产率则低约20倍。在乙腈中,ACL实际上是光稳定的。2017年9月5日进行测量的日光下的最小寿命在τ的范围内= 5-10分钟。使用平均量子产率,吸收光谱和光子通量以及对云量和装置尺寸的适当校正,可以令人满意地解释ACL在日光下的日光解。对于低浓度,在光解反应中形成的ACR的收率实际上为100%。但是,随着ACL浓度的增加,ACR的产量会下降,ACA的产量会增加。9(10 H)-A啶酮和ACM始终被检测为次要产品,收率低于2%。在ACL的任何反应中都从未检测到9-甲基ac啶。有强烈的迹象表明,ACL在位于德国下萨克森州的一条河流中发生了光解反应,并形成了等摩尔的ACR。因此,ACL是天然地表水中ACR的直接前体。
更新日期:2018-06-12
中文翻译:

水性介质中9-ac啶甲醛的光化学作用†
研究了溶液中的9-ac啶甲醛(ACL)的黑暗反应和光解反应。ACL在空气饱和溶液中,在T = 20°C和pH = 7.0的黑暗中与主要产物9-ac啶甲酸(ACA)和次要产物9-ac啶甲醇(ACM)反应,寿命τ = 4.3天。确定基本ACLH +的解离常数为p K a ± σ = 4.38±0.04。使用多色Xe光源研究了ACL的光解作用。pH> p K a时,在T = 20°C的水溶液中,在c 0(ACL)= 0.18–16.6μM的浓度范围内,量子产率对于氮气,空气和氧气充气溶液的浓度为Φ ± σ =(0.015±0.003)mol / mol。ACLH +的量子产率,即pH≪ p K a的量子产率高出2倍(Φ = 0.029 mol / mol)。与水相比,甲醇和异丙醇中的量子产率略低,而丙酮中的量子产率则低约20倍。在乙腈中,ACL实际上是光稳定的。2017年9月5日进行测量的日光下的最小寿命在τ的范围内= 5-10分钟。使用平均量子产率,吸收光谱和光子通量以及对云量和装置尺寸的适当校正,可以令人满意地解释ACL在日光下的日光解。对于低浓度,在光解反应中形成的ACR的收率实际上为100%。但是,随着ACL浓度的增加,ACR的产量会下降,ACA的产量会增加。9(10 H)-A啶酮和ACM始终被检测为次要产品,收率低于2%。在ACL的任何反应中都从未检测到9-甲基ac啶。有强烈的迹象表明,ACL在位于德国下萨克森州的一条河流中发生了光解反应,并形成了等摩尔的ACR。因此,ACL是天然地表水中ACR的直接前体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号