当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interactions of a Bacterial Cu(I)-ATPase with a Complex Lipid Environment
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-12 00:00:00 , DOI: 10.1021/acs.biochem.8b00326 Henriette E. Autzen 1, 2 , Heidi Koldsø 3 , Phillip J. Stansfeld 3 , Pontus Gourdon 4 , Mark S. P. Sansom 3 , Poul Nissen 1, 2, 5
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-12 00:00:00 , DOI: 10.1021/acs.biochem.8b00326 Henriette E. Autzen 1, 2 , Heidi Koldsø 3 , Phillip J. Stansfeld 3 , Pontus Gourdon 4 , Mark S. P. Sansom 3 , Poul Nissen 1, 2, 5
Affiliation
|
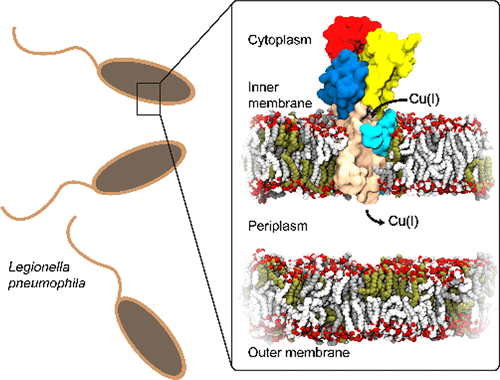
Phospholipids and sterols play multiple roles in cells. In addition to establishing barriers between compartments, they also provide the matrix for assembly and function of a large variety of catalytic processes. Lipid composition is a highly regulated feature of biological membranes, yet its implications for membrane proteins are difficult problems to approach. One obstacle is the inherent complexity of observing and describing these interactions and their dynamics at a molecular and atomic level. However, lipid interactions are pivotal for membrane protein function and should be acknowledged. The enzymatic activity of several different P-type ATPases, one of the major families of ion pumping primary active transporters, has previously been shown to exhibit a strong dependence on phospholipids; however, distinguishing the effects of annular and specific lipid interactions is challenging. Here we show that the hydrolytic activity of a bacterial Cu(I)-transporting P-type ATPase (LpCopA) is stimulated by the bacterial, anionic phospholipid cardiolipin and to some extent by phosphatidylglycerol. Furthermore, multiscale molecular dynamics simulations pinpoint lipid hot spots on the membrane-spanning domain of LpCopA. Thus, using two independent methods, our study shows converging evidence that the lipid membrane composition plays an important role for LpCopA.
中文翻译:

细菌Cu(I)-ATPase与复杂脂质环境的相互作用
磷脂和固醇在细胞中起多种作用。除了在隔室之间建立屏障之外,它们还提供了用于多种催化过程的组装和功能的基质。脂质组成是生物膜的高度调节特征,但是其对膜蛋白的影响是难以解决的问题。障碍之一是在分子和原子水平上观察和描述这些相互作用及其动力学的内在复杂性。但是,脂质相互作用对于膜蛋白功能至关重要,应予以确认。先前已显示出几种不同的P型ATP酶的酶促活性,这是离子泵浦主要活性转运蛋白的主要家族之一,对磷脂具有很强的依赖性。然而,区分环状脂质和特定脂质相互作用的作用具有挑战性。在这里,我们显示细菌铜(I)传输的P型ATP酶(LpCopA)的水解活性受到细菌阴离子磷脂心磷脂的刺激,并在一定程度上受到磷脂酰甘油的刺激。此外,多尺度分子动力学模拟可在LpCopA跨膜域上查明脂质热点。因此,使用两种独立的方法,我们的研究显示出越来越多的证据表明脂质膜成分对LpCopA起着重要的作用。多尺度分子动力学模拟可在LpCopA跨膜结构域上查明脂质热点。因此,使用两种独立的方法,我们的研究显示出越来越多的证据表明脂质膜成分对LpCopA起着重要的作用。多尺度分子动力学模拟可在LpCopA跨膜结构域上查明脂质热点。因此,使用两种独立的方法,我们的研究显示出越来越多的证据表明脂质膜成分对LpCopA起着重要的作用。
更新日期:2018-06-12
中文翻译:

细菌Cu(I)-ATPase与复杂脂质环境的相互作用
磷脂和固醇在细胞中起多种作用。除了在隔室之间建立屏障之外,它们还提供了用于多种催化过程的组装和功能的基质。脂质组成是生物膜的高度调节特征,但是其对膜蛋白的影响是难以解决的问题。障碍之一是在分子和原子水平上观察和描述这些相互作用及其动力学的内在复杂性。但是,脂质相互作用对于膜蛋白功能至关重要,应予以确认。先前已显示出几种不同的P型ATP酶的酶促活性,这是离子泵浦主要活性转运蛋白的主要家族之一,对磷脂具有很强的依赖性。然而,区分环状脂质和特定脂质相互作用的作用具有挑战性。在这里,我们显示细菌铜(I)传输的P型ATP酶(LpCopA)的水解活性受到细菌阴离子磷脂心磷脂的刺激,并在一定程度上受到磷脂酰甘油的刺激。此外,多尺度分子动力学模拟可在LpCopA跨膜域上查明脂质热点。因此,使用两种独立的方法,我们的研究显示出越来越多的证据表明脂质膜成分对LpCopA起着重要的作用。多尺度分子动力学模拟可在LpCopA跨膜结构域上查明脂质热点。因此,使用两种独立的方法,我们的研究显示出越来越多的证据表明脂质膜成分对LpCopA起着重要的作用。多尺度分子动力学模拟可在LpCopA跨膜结构域上查明脂质热点。因此,使用两种独立的方法,我们的研究显示出越来越多的证据表明脂质膜成分对LpCopA起着重要的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号