当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Structure of an As(III) S-Adenosylmethionine Methyltransferase with 3-Coordinately Bound As(III) Depicts the First Step in Catalysis
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-12 00:00:00 , DOI: 10.1021/acs.biochem.8b00457 Charles Packianathan 1 , Palani Kandavelu 2 , Barry P. Rosen 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-12 00:00:00 , DOI: 10.1021/acs.biochem.8b00457 Charles Packianathan 1 , Palani Kandavelu 2 , Barry P. Rosen 1
Affiliation
|
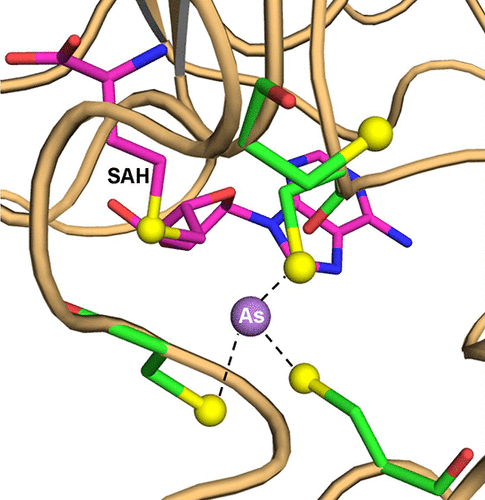
Arsenic is a ubiquitous environmental toxic substance and a Class 1 human carcinogen. Arsenic methylation by the enzyme As(III) S-adenosylmethionine (SAM) methyltransferase (ArsM in microbes or AS3MT in animals) detoxifies As(III) in microbes but transforms it into more toxic and potentially more carcinogenic methylated species in humans. We previously proposed a reaction pathway for ArsM/AS3MT that involves initial 3-coordinate binding of As(III). To date, reported structures have had only 2-coordinately bound trivalent arsenicals. Here we report a crystal structure of CmArsM from Cyanidioschyzon sp.5508 in which As(III) is 3-coordinately bound to three conserved cysteine residues with a molecule of the product S-adenosyl-l-homocysteine bound in the SAM binding site. We propose that this structure represents the first step in the catalytic cycle. In a previously reported SAM-bound structure, a disulfide bond is formed between two conserved cysteine residues. Comparison of these two structures indicates that there is a conformational change in the N-terminal domain of CmArsM that moves a loop to allow formation of the 3-coordinate As(III) binding site. We propose that this conformational change is an initial step in the As(III) SAM methyltransferase catalytic cycle.
中文翻译:

具有3位键合的As(III)的As(III)S-腺苷甲硫氨酸甲基转移酶的结构描述了催化的第一步
砷是一种普遍存在的环境有毒物质,是1类人类致癌物。As(III)S-腺苷甲硫氨酸(SAM)甲基转移酶(微生物中的ArsM或动物中的AS3MT)进行的砷甲基化使微生物中的As(III)解毒,但将其转化为人体内更具毒性和潜在致癌性的甲基化物种。我们先前提出了ArsM / AS3MT的反应途径,其中涉及As(III)的初始3坐标结合。迄今为止,报道的结构仅具有2-配位键合的三价砷。在这里,我们从报告CmArsM的晶体结构Cyanidioschyzon sp.5508其中作为(III)是三配位结合到三个保守的半胱氨酸残基与该产品的一个分子š -adenosyl-升-高半胱氨酸结合在SAM结合位点中。我们建议该结构代表催化循环的第一步。在先前报道的SAM结合结构中,在两个保守的半胱氨酸残基之间形成二硫键。这两个结构的比较表明,在CmArsM的N端结构域中存在构象变化,该构象变化使环移动以允许形成3坐标的As(III)结合位点。我们建议这种构象变化是As(III)SAM甲基转移酶催化循环的第一步。
更新日期:2018-06-12
中文翻译:

具有3位键合的As(III)的As(III)S-腺苷甲硫氨酸甲基转移酶的结构描述了催化的第一步
砷是一种普遍存在的环境有毒物质,是1类人类致癌物。As(III)S-腺苷甲硫氨酸(SAM)甲基转移酶(微生物中的ArsM或动物中的AS3MT)进行的砷甲基化使微生物中的As(III)解毒,但将其转化为人体内更具毒性和潜在致癌性的甲基化物种。我们先前提出了ArsM / AS3MT的反应途径,其中涉及As(III)的初始3坐标结合。迄今为止,报道的结构仅具有2-配位键合的三价砷。在这里,我们从报告CmArsM的晶体结构Cyanidioschyzon sp.5508其中作为(III)是三配位结合到三个保守的半胱氨酸残基与该产品的一个分子š -adenosyl-升-高半胱氨酸结合在SAM结合位点中。我们建议该结构代表催化循环的第一步。在先前报道的SAM结合结构中,在两个保守的半胱氨酸残基之间形成二硫键。这两个结构的比较表明,在CmArsM的N端结构域中存在构象变化,该构象变化使环移动以允许形成3坐标的As(III)结合位点。我们建议这种构象变化是As(III)SAM甲基转移酶催化循环的第一步。











































 京公网安备 11010802027423号
京公网安备 11010802027423号