当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Precursor-Based Selective Methyl Labeling of Cell-Free Synthesized Proteins
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-06-12 00:00:00 , DOI: 10.1021/acschembio.8b00338 Mariya Lazarova 1 , Frank Löhr 1 , Ralf-Bernhardt Rues 1 , Robin Kleebach 1 , Volker Dötsch 1 , Frank Bernhard 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-06-12 00:00:00 , DOI: 10.1021/acschembio.8b00338 Mariya Lazarova 1 , Frank Löhr 1 , Ralf-Bernhardt Rues 1 , Robin Kleebach 1 , Volker Dötsch 1 , Frank Bernhard 1
Affiliation
|
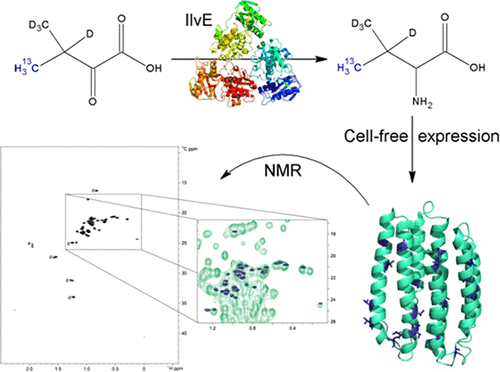
NMR studies of large proteins are complicated by pronounced spectral overlap and large line width. Reducing complexity by [13C, 1H] selective labeling of l-Val, l-Leu, and/or l-Ile residues in combination with optional perdeuteration is therefore commonly approached by supplying labeled amino acid precursors into bacterial expression cultures, although often compromised by high label costs, precursor instability, and label scrambling. Cell-free expression combines efficient production of membrane proteins with significant advantages for protein labeling such as small reaction volumes, defined amino acid pools, and reliable label incorporation. While amino acid specific isotopic labeling of proteins is routine application, the amino acid methyl side-chain labeling was so far difficult as appropriately labeled amino acids are hardly available. On the basis of recent proteome analyses of cell-free lysates, we have developed a competitive strategy for efficient methyl labeling of proteins based on conversion of supplied precursors. Pathway complexity of methyl side-chain labeling was reduced by implementing the promiscuous aminotransferase IlvE catalyzing the selective l-Leu, l-Val, or l-Ile biosynthesis from specific ketoacid precursors. Precursor-based l-Leu and l-Val synthesis was demonstrated with the cell-free labeling of peptidyl-prolyl cis/trans isomerase cyclophilin D and of the proton pump proteorhodopsin. The strategy is fast and cost-effective and enables the straightforward methyl side-chain labeling of individual amino acid types. It can easily be applied to any cell-free synthesized protein.
中文翻译:

无细胞合成蛋白的基于前体的选择性甲基标记
大蛋白的NMR研究由于明显的光谱重叠和大线宽而变得复杂。通过[ 13 C,1 H]选择性标记l -Val,l -Leu和/或l降低复杂性因此,通常通过将标记的氨基酸前体供应到细菌表达培养物中来实现与残基结合任选的氘化的作用,尽管通常会因高标记成本,前体不稳定和标记混乱而受到损害。无细胞表达将膜蛋白的高效生产与蛋白标记的显着优势相结合,例如较小的反应体积,确定的氨基酸库和可靠的标记掺入。虽然蛋白质的氨基酸特异性同位素标记是常规应用,但由于很难获得经过适当标记的氨基酸,因此氨基酸甲基侧链标记至今仍很困难。根据最近对无细胞裂解物的蛋白质组分析,我们已经开发了一种竞争策略,可以根据所提供的前体转化对蛋白质进行有效的甲基标记。通过实施混杂的氨基转移酶IlvE催化选择性的甲基化,降低了甲基侧链标记的途径复杂性升-Leu,升-Val或升来自特定生物合成-Ile酮酸前体。用肽基-脯氨酰基顺/反异构酶亲环蛋白D和质子泵蛋白视紫红质的无细胞标记证明了基于前体的1 - Leu和1- Val合成。该策略是快速且具有成本效益的,并且能够对单个氨基酸类型进行直接的甲基侧链标记。它可以轻松地应用于任何无细胞合成蛋白。
更新日期:2018-06-12
中文翻译:

无细胞合成蛋白的基于前体的选择性甲基标记
大蛋白的NMR研究由于明显的光谱重叠和大线宽而变得复杂。通过[ 13 C,1 H]选择性标记l -Val,l -Leu和/或l降低复杂性因此,通常通过将标记的氨基酸前体供应到细菌表达培养物中来实现与残基结合任选的氘化的作用,尽管通常会因高标记成本,前体不稳定和标记混乱而受到损害。无细胞表达将膜蛋白的高效生产与蛋白标记的显着优势相结合,例如较小的反应体积,确定的氨基酸库和可靠的标记掺入。虽然蛋白质的氨基酸特异性同位素标记是常规应用,但由于很难获得经过适当标记的氨基酸,因此氨基酸甲基侧链标记至今仍很困难。根据最近对无细胞裂解物的蛋白质组分析,我们已经开发了一种竞争策略,可以根据所提供的前体转化对蛋白质进行有效的甲基标记。通过实施混杂的氨基转移酶IlvE催化选择性的甲基化,降低了甲基侧链标记的途径复杂性升-Leu,升-Val或升来自特定生物合成-Ile酮酸前体。用肽基-脯氨酰基顺/反异构酶亲环蛋白D和质子泵蛋白视紫红质的无细胞标记证明了基于前体的1 - Leu和1- Val合成。该策略是快速且具有成本效益的,并且能够对单个氨基酸类型进行直接的甲基侧链标记。它可以轻松地应用于任何无细胞合成蛋白。











































 京公网安备 11010802027423号
京公网安备 11010802027423号