Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2018-06-12 , DOI: 10.1016/j.bioorg.2018.06.018 Savvas Thysiadis , Sotirios Katsamakas , Spyros Mpousis , Nicolaos Avramidis , Spiros Efthimiopoulos , Vasiliki Sarli
|
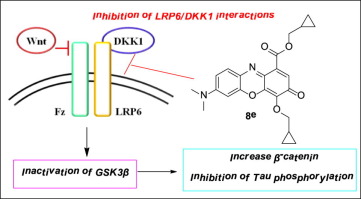
Based on NCI8642, a series of gallocyanine derivatives was synthesized with modifications of the substituent groups in position 1, 2 and 4 of the phenoxazinone scaffold. The effectiveness of gallocyanines to inhibit DKK1/LRP6 interactions and Tau phosphorylation induced by prostaglandin J2 and DKK1 was elucidated by both experimental data and molecular docking simulations. Bis-alkylated with flexible alkyl ester groups on C1 and bis-benzyl gallocyanines provided the most active inhibitors, while amino derivatives on C2 of NCI8642 that have alkoxy or benzyloxy substituents on C4, were less active. Furthermore, it is shown that treating of SHSY5Y cells with NCI8642 derivatives activates Wnt signaling and increases the levels of pGSK3β kinase and β-catenin.
中文翻译:

用于治疗阿尔茨海默氏病的DKK1 / LRP6相互作用的花青素抑制剂的设计与合成
基于NCI8642,合成了一系列的花青衍生物,其修饰了苯恶嗪酮骨架的1、2和4位的取代基。实验数据和分子对接模拟均阐明了花青素抑制前列腺素J2和DKK1诱导的DKK1 / LRP6相互作用和Tau磷酸化的有效性。用C1和双苄基没食子花青素上的柔性烷基酯基进行双烷基化可提供最具活性的抑制剂,而NCI8642的C2上具有C4上的烷氧基或苄氧基取代基的氨基衍生物则活性较低。此外,显示了用NCI8642衍生物处理SHSY5Y细胞激活了Wnt信号传导并增加了pGSK3β激酶和β-连环蛋白的水平。











































 京公网安备 11010802027423号
京公网安备 11010802027423号