当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
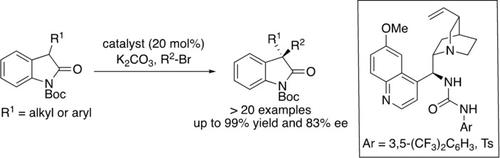
Catalytic and Enantioselective Direct α‐Alkylation of 3‐Aryl and 3‐Alkyl Oxindole Using Quinine‐Derived Urea Catalyst
ChemistrySelect ( IF 2.1 ) Pub Date : 2018-06-12 , DOI: 10.1002/slct.201800906 Monissa C. Paderes 1, 2 , Woon Yew Siau 1 , Ziqiang Rong 1 , Yu Zhao 1
ChemistrySelect ( IF 2.1 ) Pub Date : 2018-06-12 , DOI: 10.1002/slct.201800906 Monissa C. Paderes 1, 2 , Woon Yew Siau 1 , Ziqiang Rong 1 , Yu Zhao 1
Affiliation
|
We report herein an enantioselective direct α‐alkylation of various substituted oxindole derivatives using simple alkyl bromide and a bifunctional quinine‐derived urea catalyst. The developed method catalyzes the α‐alkylation of both 3‐aryl and 3‐alkyl substituted oxindole. The alkylated oxindole products, which contain a chiral quaternary stereocenter were afforded in excellent yields (up to 99%) and moderate to good enantioselectivity (up to 83%).
中文翻译:

奎宁衍生的尿素催化剂催化3-芳基和3-烷基氧吲哚的催化和对映选择性直接α-烷基化
我们在这里报告了使用简单的烷基溴和双官能奎宁衍生的尿素催化剂对各种取代的羟吲哚衍生物的对映选择性直接α-烷基化反应。所开发的方法催化3-芳基和3-烷基取代的羟吲哚的α-烷基化反应。含有手性季立体中心的烷基化羟吲哚产物以优异的收率(高达99%)和中等至良好的对映选择性(高达83%)提供。
更新日期:2018-06-12
中文翻译:

奎宁衍生的尿素催化剂催化3-芳基和3-烷基氧吲哚的催化和对映选择性直接α-烷基化
我们在这里报告了使用简单的烷基溴和双官能奎宁衍生的尿素催化剂对各种取代的羟吲哚衍生物的对映选择性直接α-烷基化反应。所开发的方法催化3-芳基和3-烷基取代的羟吲哚的α-烷基化反应。含有手性季立体中心的烷基化羟吲哚产物以优异的收率(高达99%)和中等至良好的对映选择性(高达83%)提供。



























 京公网安备 11010802027423号
京公网安备 11010802027423号