当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Post-Synthetic Modification of Oligonucleotides via Orthogonal Amidation and Copper Catalyzed Cycloaddition Reactions
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-06-12 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00298 Daniel Zewge 1 , Gabor Butora 1 , Edward C. Sherer 1 , David M. Tellers , Daniel R. Sidler 1 , Joseph Gouker 1 , Greg Copeland 1 , Vasant Jadhav , Zhen Li 1 , Joseph Armstrong 1 , Ian W. Davies 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-06-12 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00298 Daniel Zewge 1 , Gabor Butora 1 , Edward C. Sherer 1 , David M. Tellers , Daniel R. Sidler 1 , Joseph Gouker 1 , Greg Copeland 1 , Vasant Jadhav , Zhen Li 1 , Joseph Armstrong 1 , Ian W. Davies 1
Affiliation
|
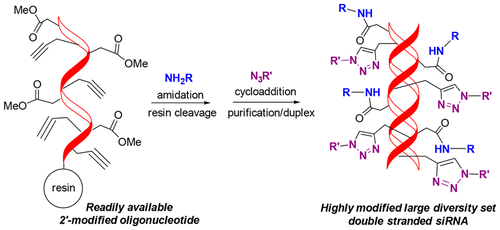
An efficient multicomponent orthogonal protocol was developed for post-synthetic oligonucleotide modification using commercially available 2′-O-methyl ester and 2′-O-propargyl nucleoside scaffolds. Amidation of methyl esters with primary amines was achieved in the presence of 2′-propargyl groups which were utilized for subsequent copper catalyzed cycloaddition with GalNAc-azide. The methodology was applied to generate siRNA composed of multiple amide and triazole conjugates. Computational methods were used to illustrate the impact of substitution at the 2′-position. This a powerful post-oligomerization technique for rapidly introducing diversity to oligonucleotide design.
中文翻译:

通过正交酰胺化和铜催化的环加成反应对寡核苷酸进行合成后修饰
开发了一种有效的多组分正交方案,用于使用市售的2'- O-甲酯和2'- O-炔丙基核苷支架进行合成后的寡核苷酸修饰。在存在2'-炔丙基的情况下实现了甲基酯与伯胺的酰胺化,该芳基用于随后的铜与GalNAc-叠氮化物催化的环加成反应。该方法已应用于生成由多种酰胺和三唑共轭物组成的siRNA。使用计算方法来说明取代在2'位置的影响。这种强大的低聚后技术,可快速将多样性引入寡核苷酸设计。
更新日期:2018-06-12
中文翻译:

通过正交酰胺化和铜催化的环加成反应对寡核苷酸进行合成后修饰
开发了一种有效的多组分正交方案,用于使用市售的2'- O-甲酯和2'- O-炔丙基核苷支架进行合成后的寡核苷酸修饰。在存在2'-炔丙基的情况下实现了甲基酯与伯胺的酰胺化,该芳基用于随后的铜与GalNAc-叠氮化物催化的环加成反应。该方法已应用于生成由多种酰胺和三唑共轭物组成的siRNA。使用计算方法来说明取代在2'位置的影响。这种强大的低聚后技术,可快速将多样性引入寡核苷酸设计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号