当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of Hydrophobicity and Charge of Amyloid-Beta Oligomer Eliminating d-Peptides in the Interaction with Amyloid-Beta Monomers.
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-06-21 , DOI: 10.1021/acschemneuro.8b00132 Tamar Ziehm 1 , Alexander K Buell 2 , Dieter Willbold 1, 2
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-06-21 , DOI: 10.1021/acschemneuro.8b00132 Tamar Ziehm 1 , Alexander K Buell 2 , Dieter Willbold 1, 2
Affiliation
|
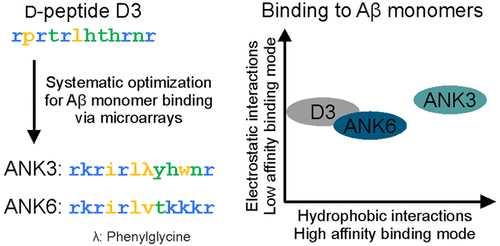
Inhibition of the self-assembly process of amyloid-beta and even more the removal of already existing toxic amyloid-beta assemblies represent promising therapeutic strategies against Alzheimer's disease. To approach this aim, we selected a d-enantiomeric peptide by phage-display based on the interaction with amyloid-beta monomers. This lead compound was successfully optimized by peptide microarrays with respect to its affinity and specificity to the target resulting in d-peptides with both increased hydrophobicity and net charge. Here, we present a detailed biophysical characterization of the interactions between these optimized d-peptides and amyloid-beta monomers in comparison to the original lead compound in order to obtain a more thorough understanding of the physicochemical determinants of the interactions. Kinetics and apparent stoichiometry of complex formation were studied using surface plasmon resonance. Potential modes of binding to amyloid-beta were identified, and the influences of ionic strength on complex stability, as well as on the inhibitory effect on amyloid-beta aggregation were investigated. The results reveal a very different mode of interaction of the optimized d-peptides based on a combination of electrostatic and hydrophobic interactions as compared to the mostly electrostatically driven interaction of the lead compound. These conclusions were supported by the thermodynamic profiles of the interaction between optimized d-peptides and Aβ monomers, which indicate an increase in binding entropy with respect to the lead compound.
中文翻译:

淀粉样-β寡聚物消除d肽的疏水性和电荷的作用在与淀粉样-β单体的相互作用中。
抑制淀粉样蛋白β的自组装过程,甚至去除已经存在的有毒淀粉样β蛋白的组装代表了针对阿尔茨海默氏病的有前途的治疗策略。为了达到这个目的,我们基于与淀粉状蛋白-β单体的相互作用,通过噬菌体展示选择了一种d-对映体肽。通过肽微阵列就其对靶标的亲和力和特异性成功地优化了该先导化合物,从而得到了具有增加的疏水性和净电荷的d-肽。在这里,我们提出了与原始的先导化合物相比,这些优化的d肽和淀粉样β单体之间的相互作用的详细生物物理表征,以便更全面地了解相互作用的物理化学决定因素。使用表面等离振子共振研究了复合物形成的动力学和表观化学计量。确定了与淀粉样蛋白β结合的潜在模式,并研究了离子强度对复合物稳定性以及对淀粉样蛋白β聚集的抑制作用的影响。结果表明,与主要由静电驱动的前导化合物相互作用相比,基于静电和疏水相互作用的组合,优化的d肽的相互作用模式非常不同。优化的d肽与Aβ单体之间相互作用的热力学曲线支持了这些结论,这表明相对于先导化合物,结合熵增加。确定了与淀粉样蛋白β结合的潜在模式,并研究了离子强度对复合物稳定性以及对淀粉样蛋白β聚集的抑制作用的影响。结果表明,与主要由静电驱动的前导化合物相互作用相比,基于静电和疏水相互作用的组合,优化的d肽的相互作用模式非常不同。优化的d肽与Aβ单体之间相互作用的热力学曲线支持了这些结论,这表明相对于先导化合物,结合熵增加。确定了与淀粉样蛋白β结合的潜在模式,并研究了离子强度对复合物稳定性以及对淀粉样蛋白β聚集的抑制作用的影响。结果表明,与主要由静电驱动的前导化合物相互作用相比,基于静电和疏水相互作用的组合,优化的d肽的相互作用模式非常不同。优化的d肽与Aβ单体之间相互作用的热力学曲线支持了这些结论,这表明相对于先导化合物,结合熵增加。结果表明,与主要由静电驱动的前导化合物相互作用相比,基于静电和疏水相互作用的组合,优化的d肽的相互作用模式非常不同。优化的d肽与Aβ单体之间相互作用的热力学曲线支持了这些结论,这表明相对于先导化合物,结合熵增加。结果表明,与主要由静电驱动的前导化合物相互作用相比,基于静电和疏水相互作用的组合,优化的d肽的相互作用模式非常不同。优化的d肽与Aβ单体之间相互作用的热力学曲线支持了这些结论,这表明相对于先导化合物,结合熵增加了。
更新日期:2018-06-12
中文翻译:

淀粉样-β寡聚物消除d肽的疏水性和电荷的作用在与淀粉样-β单体的相互作用中。
抑制淀粉样蛋白β的自组装过程,甚至去除已经存在的有毒淀粉样β蛋白的组装代表了针对阿尔茨海默氏病的有前途的治疗策略。为了达到这个目的,我们基于与淀粉状蛋白-β单体的相互作用,通过噬菌体展示选择了一种d-对映体肽。通过肽微阵列就其对靶标的亲和力和特异性成功地优化了该先导化合物,从而得到了具有增加的疏水性和净电荷的d-肽。在这里,我们提出了与原始的先导化合物相比,这些优化的d肽和淀粉样β单体之间的相互作用的详细生物物理表征,以便更全面地了解相互作用的物理化学决定因素。使用表面等离振子共振研究了复合物形成的动力学和表观化学计量。确定了与淀粉样蛋白β结合的潜在模式,并研究了离子强度对复合物稳定性以及对淀粉样蛋白β聚集的抑制作用的影响。结果表明,与主要由静电驱动的前导化合物相互作用相比,基于静电和疏水相互作用的组合,优化的d肽的相互作用模式非常不同。优化的d肽与Aβ单体之间相互作用的热力学曲线支持了这些结论,这表明相对于先导化合物,结合熵增加。确定了与淀粉样蛋白β结合的潜在模式,并研究了离子强度对复合物稳定性以及对淀粉样蛋白β聚集的抑制作用的影响。结果表明,与主要由静电驱动的前导化合物相互作用相比,基于静电和疏水相互作用的组合,优化的d肽的相互作用模式非常不同。优化的d肽与Aβ单体之间相互作用的热力学曲线支持了这些结论,这表明相对于先导化合物,结合熵增加。确定了与淀粉样蛋白β结合的潜在模式,并研究了离子强度对复合物稳定性以及对淀粉样蛋白β聚集的抑制作用的影响。结果表明,与主要由静电驱动的前导化合物相互作用相比,基于静电和疏水相互作用的组合,优化的d肽的相互作用模式非常不同。优化的d肽与Aβ单体之间相互作用的热力学曲线支持了这些结论,这表明相对于先导化合物,结合熵增加。结果表明,与主要由静电驱动的前导化合物相互作用相比,基于静电和疏水相互作用的组合,优化的d肽的相互作用模式非常不同。优化的d肽与Aβ单体之间相互作用的热力学曲线支持了这些结论,这表明相对于先导化合物,结合熵增加。结果表明,与主要由静电驱动的前导化合物相互作用相比,基于静电和疏水相互作用的组合,优化的d肽的相互作用模式非常不同。优化的d肽与Aβ单体之间相互作用的热力学曲线支持了这些结论,这表明相对于先导化合物,结合熵增加了。











































 京公网安备 11010802027423号
京公网安备 11010802027423号