当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of the Norhasubanan Alkaloid Stephadiamine
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-06-11 , DOI: 10.1021/jacs.8b01918 Nina Hartrampf 1 , Nils Winter 1 , Gabriele Pupo 2 , Brian M. Stoltz 3 , Dirk Trauner 1, 4
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-06-11 , DOI: 10.1021/jacs.8b01918 Nina Hartrampf 1 , Nils Winter 1 , Gabriele Pupo 2 , Brian M. Stoltz 3 , Dirk Trauner 1, 4
Affiliation
|
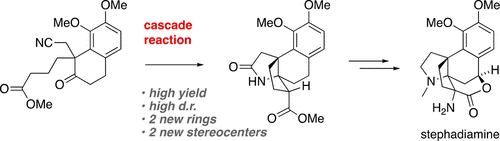
(+)-Stephadiamine is an unusual alkaloid isolated from the vine Stephania japonica. It features a norhasubanan skeleton, and contains two adjacent α-tertiary amines, which renders it an attractive synthetic target. Here, we present the first total synthesis of stephadiamine, which hinges on an efficient cascade reaction to implement the aza[4.3.3]propellane core of the alkaloid. The α-aminolactone moiety in a highly hindered position was installed via Tollens reaction and Curtius rearrangement. Useful building blocks for the asymmetric synthesis of morphine and (nor)hasubanan alkaloids are introduced.
中文翻译:

Norhasubanan 生物碱 Stephadiamine 的全合成
(+)-Stephadiamine 是一种不寻常的生物碱,从藤本植物 Stephania japonica 中分离出来。它具有norhasubanan 骨架,并含有两个相邻的α-叔胺,这使其成为有吸引力的合成目标。在这里,我们展示了第一个全合成 Stephadiamine,这取决于有效的级联反应来实现生物碱的氮杂 [4.3.3] 推进剂核心。通过 Tollens 反应和 Curtius 重排安装了高度受阻位置的 α-氨基内酯部分。介绍了用于不对称合成吗啡和(nor)hasubanan 生物碱的有用构件。
更新日期:2018-06-11
中文翻译:

Norhasubanan 生物碱 Stephadiamine 的全合成
(+)-Stephadiamine 是一种不寻常的生物碱,从藤本植物 Stephania japonica 中分离出来。它具有norhasubanan 骨架,并含有两个相邻的α-叔胺,这使其成为有吸引力的合成目标。在这里,我们展示了第一个全合成 Stephadiamine,这取决于有效的级联反应来实现生物碱的氮杂 [4.3.3] 推进剂核心。通过 Tollens 反应和 Curtius 重排安装了高度受阻位置的 α-氨基内酯部分。介绍了用于不对称合成吗啡和(nor)hasubanan 生物碱的有用构件。











































 京公网安备 11010802027423号
京公网安备 11010802027423号