当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of Boron and Phosphorus in Enhanced Electrocatalytic Oxygen Evolution by Nickel Borides and Nickel Phosphides
ChemElectroChem ( IF 3.5 ) Pub Date : 2018-06-21 , DOI: 10.1002/celc.201800669 Justus Masa 1 , Corina Andronescu 1 , Hendrik Antoni 2 , Ilya Sinev 3 , Sabine Seisel 1 , Karina Elumeeva 1 , Stefan Barwe 1 , Sara Marti-Sanchez 4 , Jordi Arbiol 4, 5 , Beatriz Roldan Cuenya 3, 6 , Martin Muhler 3 , Wolfgang Schuhmann 1
ChemElectroChem ( IF 3.5 ) Pub Date : 2018-06-21 , DOI: 10.1002/celc.201800669 Justus Masa 1 , Corina Andronescu 1 , Hendrik Antoni 2 , Ilya Sinev 3 , Sabine Seisel 1 , Karina Elumeeva 1 , Stefan Barwe 1 , Sara Marti-Sanchez 4 , Jordi Arbiol 4, 5 , Beatriz Roldan Cuenya 3, 6 , Martin Muhler 3 , Wolfgang Schuhmann 1
Affiliation
|
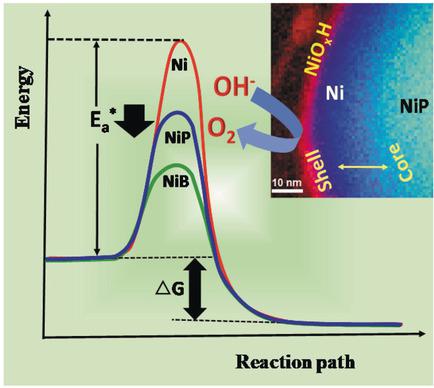
The modification of nickel with boron or phosphorus leads to significant enhancement of its electrocatalytic activity for the oxygen evolution reaction (OER). However, the precise role of the guest elements, B and P, in enhancing the OER of the host element (Ni) remains unclear. Herein, we present insight into the role of B and P in enhancing electrocatalysis of oxygen evolution by nickel borides and nickel phosphides. The apparent activation energy, Ea*, of electrocatalytic oxygen evolution on Ni2P was 78.4 kJ/mol, on Ni2B 65.4 kJ/mol, and on Ni nanoparticles 94.0 kJ/mol, thus revealing that both B and P affect the intrinsic activity of nickel. XPS data revealed shifts of −0.30 and 0.40 eV in the binding energy of the Ni 2p3/2 peak of Ni2B and Ni2P, respectively, with respect to that of pure Ni at 852.60 eV, thus indicating that B and P induce opposite electronic effects on the surface electronic structure of Ni. The origin of enhanced activity for oxygen evolution cannot, therefore, be attributed to such electronic modification or ligand effect. Severe changes induced on the nickel lattice, specifically, the Ni‐Ni atomic order and interatomic distances (strain effect), by the presence of the guest atoms seem to be the dominant factors responsible for enhanced activity of oxygen evolution in nickel borides and nickel phosphides.
中文翻译:

硼和磷在硼化镍和磷化镍增强电催化氧气释放中的作用
用硼或磷对镍进行改性可显着提高其对氧释放反应(OER)的电催化活性。但是,尚不清楚客体元素B和P在增强主体元素(Ni)的OER中的确切作用。在本文中,我们介绍了B和P在增强硼化镍和磷化镍对氧气析出的电催化作用中的作用。Ni 2 P上的电催化氧逸出的表观活化能E a *为78.4 kJ / mol,Ni 2 B上的表观活化能为65.4 kJ / mol,Ni纳米颗粒上的表观活化能为94.0 kJ / mol。镍的固有活性。XPS数据显示Ni 2p 3/2的结合能发生了-0.30和0.40 eV的变化相对于纯Ni在852.60 eV处的Ni 2 B和Ni 2 P峰,表明B和P对Ni的表面电子结构产生相反的电子效应。因此,放氧活性增强的起源不能归因于这种电子修饰或配体效应。客体原子的存在在镍晶格上引起的剧烈变化,特别是Ni-Ni原子序和原子间距离(应变效应)似乎是导致硼化镍和磷化镍中氧释放活性增强的主要因素。 。
更新日期:2018-06-21
中文翻译:

硼和磷在硼化镍和磷化镍增强电催化氧气释放中的作用
用硼或磷对镍进行改性可显着提高其对氧释放反应(OER)的电催化活性。但是,尚不清楚客体元素B和P在增强主体元素(Ni)的OER中的确切作用。在本文中,我们介绍了B和P在增强硼化镍和磷化镍对氧气析出的电催化作用中的作用。Ni 2 P上的电催化氧逸出的表观活化能E a *为78.4 kJ / mol,Ni 2 B上的表观活化能为65.4 kJ / mol,Ni纳米颗粒上的表观活化能为94.0 kJ / mol。镍的固有活性。XPS数据显示Ni 2p 3/2的结合能发生了-0.30和0.40 eV的变化相对于纯Ni在852.60 eV处的Ni 2 B和Ni 2 P峰,表明B和P对Ni的表面电子结构产生相反的电子效应。因此,放氧活性增强的起源不能归因于这种电子修饰或配体效应。客体原子的存在在镍晶格上引起的剧烈变化,特别是Ni-Ni原子序和原子间距离(应变效应)似乎是导致硼化镍和磷化镍中氧释放活性增强的主要因素。 。











































 京公网安备 11010802027423号
京公网安备 11010802027423号