当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Hydrogen Evolution Reaction Efficiently Catalyzed by Ru2P Nanoparticles
ChemSusChem ( IF 7.5 ) Pub Date : 2018-07-12 , DOI: 10.1002/cssc.201801103 Yuan Wang 1 , Zong Liu 1 , Hui Liu 1 , Nian‐Tzu Suen 1 , Xu Yu 1 , Ligang Feng 1
ChemSusChem ( IF 7.5 ) Pub Date : 2018-07-12 , DOI: 10.1002/cssc.201801103 Yuan Wang 1 , Zong Liu 1 , Hui Liu 1 , Nian‐Tzu Suen 1 , Xu Yu 1 , Ligang Feng 1
Affiliation
|
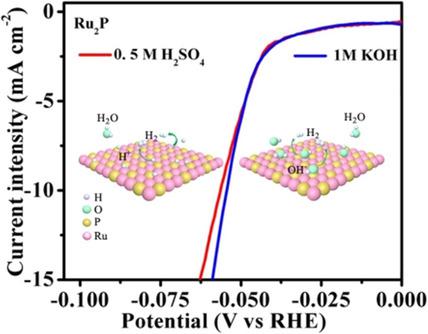
Developing alternatives to Pt catalysts is a prerequisite to cost‐effectively produce hydrogen. Herein, we demonstrate Ru2P nanoparticles (without any doping and modifications) as a highly efficient Pt‐like catalyst for the hydrogen evolution reaction (HER) in different pH electrolytes. On transferring the hexagonal close‐packed crystal structure of Ru to the orthorhombic structure of Ru2P, a greatly improved catalytic activity and stability toward HER is found owing to Ru−P coordination. The electronic state change originates from the P−Ru bonding structures, which accounts for the HER activity improvement compared with Ru nanoparticles. Specifically, Ru2P nanoparticles can drive 10 mA cm−2 at a very low overpotential of 55 mV, only 8 mV more than Pt/C in an acidic solution; and an extremely low overpotential of approximately 50 mV is needed in alkaline solution, about 20 mV less than the Pt/C catalyst. The Volmer–Tafel mechanism is indicated on Ru2P nanoparticles with the typical Tafel slope of 30 mV dec−1 of Pt metal indicating a Pt‐like catalytic ability. Ru2P is more active in the Ru‐P family as H atoms prefer to adsorb on Ru atoms rather than on the P element according to theoretical calculations. Considering the low price of Ru (20 % of Pt), anti‐corrosion ability in the electrolyte, and the safe and reliable fabrication approach, the powder Ru2P nanoparticles make an excellent HER catalyst with great promise for large‐scale water electrolysis applications.
中文翻译:

Ru2P纳米粒子有效催化电化学放氢反应
开发Pt催化剂的替代品是经济高效地生产氢气的先决条件。本文中,我们证明了Ru 2 P纳米颗粒(无任何掺杂和修饰)是一种在不同pH值的电解液中用于氢释放反应(HER)的高效Pt状催化剂。通过将Ru的六方紧密堆积晶体结构转移到Ru 2 P的正交晶体结构,由于Ru-P配位,催化活性和对HER的稳定性大大提高。电子状态变化源自P-Ru键结构,与Ru纳米颗粒相比,这说明HER活性有所提高。具体而言,Ru 2 P纳米颗粒可以驱动10 mA cm -2在55 mV的极低电势下,仅比酸性溶液中的Pt / C高8 mV;碱性溶液需要极低的过电势,约为50 mV,比Pt / C催化剂低约20 mV。Volmer-Tafel机制在Ru 2 P纳米颗粒上显示出来,其典型的Tafel斜率是Pt金属的30 mV dec -1,表明了类似Pt的催化能力。Ru 2 P在Ru‐P族中更具活性,因为根据理论计算,H原子更喜欢吸附在Ru原子上而不是P元素上。考虑到Ru的价格低廉(Pt的20%),电解质的抗腐蚀能力以及安全可靠的制造方法,Ru 2粉末P纳米粒子是一种出色的HER催化剂,对于大规模水电解应用具有广阔的前景。
更新日期:2018-07-12
中文翻译:

Ru2P纳米粒子有效催化电化学放氢反应
开发Pt催化剂的替代品是经济高效地生产氢气的先决条件。本文中,我们证明了Ru 2 P纳米颗粒(无任何掺杂和修饰)是一种在不同pH值的电解液中用于氢释放反应(HER)的高效Pt状催化剂。通过将Ru的六方紧密堆积晶体结构转移到Ru 2 P的正交晶体结构,由于Ru-P配位,催化活性和对HER的稳定性大大提高。电子状态变化源自P-Ru键结构,与Ru纳米颗粒相比,这说明HER活性有所提高。具体而言,Ru 2 P纳米颗粒可以驱动10 mA cm -2在55 mV的极低电势下,仅比酸性溶液中的Pt / C高8 mV;碱性溶液需要极低的过电势,约为50 mV,比Pt / C催化剂低约20 mV。Volmer-Tafel机制在Ru 2 P纳米颗粒上显示出来,其典型的Tafel斜率是Pt金属的30 mV dec -1,表明了类似Pt的催化能力。Ru 2 P在Ru‐P族中更具活性,因为根据理论计算,H原子更喜欢吸附在Ru原子上而不是P元素上。考虑到Ru的价格低廉(Pt的20%),电解质的抗腐蚀能力以及安全可靠的制造方法,Ru 2粉末P纳米粒子是一种出色的HER催化剂,对于大规模水电解应用具有广阔的前景。











































 京公网安备 11010802027423号
京公网安备 11010802027423号