当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
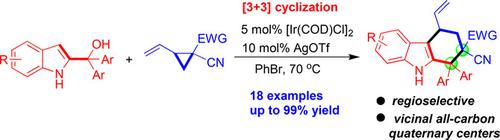
Regioselective [3+3] Cyclization of 2‐Indolymethanols with Vinylcyclopropanes via Metal Catalysis
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-07-04 , DOI: 10.1002/adsc.201800688 Zi-Qi Zhu 1 , Lei Yu 1 , Meng Sun 1 , Guang-Jian Mei 1 , Feng Shi 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-07-04 , DOI: 10.1002/adsc.201800688 Zi-Qi Zhu 1 , Lei Yu 1 , Meng Sun 1 , Guang-Jian Mei 1 , Feng Shi 1
Affiliation
|
The first [3+3] cyclization of 2‐indolylmethanols with vinylcyclopropanes has been established under iridium catalysis. The reaction regioselectively constructs tetrahydrocarbazole frameworks bearing two vicinal all‐carbon quaternary stereocenters in high yields (up to 99%, up to 20:1 rr). This reaction not only is the first example of a metal‐catalyzed [3+3] cyclization of 2‐indolylmethanols but also is the first one‐step [3+3] cyclization of vinylcyclopropanes. More importantly, this reaction shows reversed regioselectivity compared to other 2‐indolylmethanol‐involved transformations, which will advance the chemistry of 2‐indolylmethanols.
中文翻译:

通过金属催化与乙烯基环丙烷的2-吲哚甲醇的区域选择性[3 + 3]环化
在铱催化下,已经建立了2-吲哚基甲醇与乙烯基环丙烷的第一个[3 + 3]环化反应。该反应区域选择性地以高产率(高达99%,高达20:1 rr)构建带有两个邻近的全碳四元立体中心的四氢咔唑骨架。该反应不仅是2-吲哚基甲醇在金属催化下[3 + 3]环化的第一个例子,而且还是乙烯基环丙烷的第一步[3 + 3]环化的例子。更重要的是,与其他2吲哚基甲醇参与的转化相比,该反应显示出反向的区域选择性,这将促进2吲哚基甲醇的化学反应。
更新日期:2018-07-04
中文翻译:

通过金属催化与乙烯基环丙烷的2-吲哚甲醇的区域选择性[3 + 3]环化
在铱催化下,已经建立了2-吲哚基甲醇与乙烯基环丙烷的第一个[3 + 3]环化反应。该反应区域选择性地以高产率(高达99%,高达20:1 rr)构建带有两个邻近的全碳四元立体中心的四氢咔唑骨架。该反应不仅是2-吲哚基甲醇在金属催化下[3 + 3]环化的第一个例子,而且还是乙烯基环丙烷的第一步[3 + 3]环化的例子。更重要的是,与其他2吲哚基甲醇参与的转化相比,该反应显示出反向的区域选择性,这将促进2吲哚基甲醇的化学反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号