当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction Mechanisms of Sodium‐Ion Batteries under Various Charge and Discharge Conditions in a Three‐Electrode Setup
ChemElectroChem ( IF 3.5 ) Pub Date : 2018-06-27 , DOI: 10.1002/celc.201800387 Xiaona Song 1 , Xunfu Zhou 2 , Yanxue Zhou 3 , Yaoming Deng 1 , Tao Meng 1 , Aimei Gao 1 , Junmin Nan 1 , Dong Shu 1, 4, 5 , Fenyun Yi 1
ChemElectroChem ( IF 3.5 ) Pub Date : 2018-06-27 , DOI: 10.1002/celc.201800387 Xiaona Song 1 , Xunfu Zhou 2 , Yanxue Zhou 3 , Yaoming Deng 1 , Tao Meng 1 , Aimei Gao 1 , Junmin Nan 1 , Dong Shu 1, 4, 5 , Fenyun Yi 1
Affiliation
|
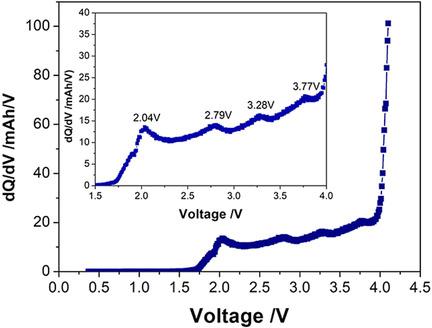
Charge‐discharge reaction mechanisms of sodium‐ion batteries under various condition are studied by using a three‐electrode setup of a pouch‐type sodium‐ion battery. The sodium‐ion battery is constructed by using cost‐effective ternary layered Na0.76Ni0.3Fe0.4Mn0.3O2 and commercial hard carbon as the cathode and anode materials, respectively, and 1.0 M NaPF6 in mixed carbonate solvent as the electrolyte. The electrochemical impedance spectroscopy (EIS) results and transmission electron microscopy (TEM) images show that an apparent solid‐electrolyte interphase (SEI) film is formed on anode material surface during the formation (pre‐charging) process, and the potentials for the SEI film formation of the solvents (ethylene carbonate and ethylene carbonate) and the additive (fluorinated ethylene carbonate) in the electrolyte are 2.04 and 2.79 V, respectively. The X‐ray diffraction (XRD) results demonstrate that, during charge, the crystal structure of cathode material changes significantly with the deintercalation of Na+. When the battery is charged to 5.0 V, the diffraction peak corresponding to the (002) plane disappears, as Na+ is further deintercalated, and the structure changes from the hexagonal phase to the monoclinic phase, causing the rapid degradation of the cycle performance. When the battery is overdischarged to 0 V, the EIS results and TEM images show that the SEI film is destroyed completely, and the cycle life performance is significantly deteriorated.
中文翻译:

三电极设置下各种充电和放电条件下钠离子电池的反应机理
通过使用袋式钠离子电池的三电极设置,研究了在各种条件下钠离子电池的充放电反应机理。钠离子电池是通过使用经济高效的三元层状Na 0.76 Ni 0.3 Fe 0.4 Mn 0.3 O 2和商用硬碳分别作为正极和负极材料以及1.0 M NaPF 6制成的在混合碳酸盐溶剂中作电解质。电化学阻抗谱(EIS)结果和透射电子显微镜(TEM)图像显示,在形成(预充电)过程中,阳极材料表面形成了明显的固体-电解质中间相(SEI)膜,并且存在SEI的电势电解质中溶剂(碳酸亚乙酯和碳酸亚乙酯)和添加剂(氟化碳酸亚乙酯)的成膜分别为2.04和2.79V。X射线衍射(XRD)结果表明,在充电过程中,阴极材料的晶体结构会随着Na +的脱嵌而发生显着变化。当电池充电至5.0 V时,对应于(002)平面的衍射峰消失,因为Na +进一步脱嵌,结构从六方相变为单斜晶相,导致循环性能迅速下降。当电池过放电至0 V时,EIS结果和TEM图像显示SEI膜被完全破坏,循环寿命性能显着下降。
更新日期:2018-06-27
中文翻译:

三电极设置下各种充电和放电条件下钠离子电池的反应机理
通过使用袋式钠离子电池的三电极设置,研究了在各种条件下钠离子电池的充放电反应机理。钠离子电池是通过使用经济高效的三元层状Na 0.76 Ni 0.3 Fe 0.4 Mn 0.3 O 2和商用硬碳分别作为正极和负极材料以及1.0 M NaPF 6制成的在混合碳酸盐溶剂中作电解质。电化学阻抗谱(EIS)结果和透射电子显微镜(TEM)图像显示,在形成(预充电)过程中,阳极材料表面形成了明显的固体-电解质中间相(SEI)膜,并且存在SEI的电势电解质中溶剂(碳酸亚乙酯和碳酸亚乙酯)和添加剂(氟化碳酸亚乙酯)的成膜分别为2.04和2.79V。X射线衍射(XRD)结果表明,在充电过程中,阴极材料的晶体结构会随着Na +的脱嵌而发生显着变化。当电池充电至5.0 V时,对应于(002)平面的衍射峰消失,因为Na +进一步脱嵌,结构从六方相变为单斜晶相,导致循环性能迅速下降。当电池过放电至0 V时,EIS结果和TEM图像显示SEI膜被完全破坏,循环寿命性能显着下降。











































 京公网安备 11010802027423号
京公网安备 11010802027423号