Journal of Functional Foods ( IF 3.8 ) Pub Date : 2018-06-09 , DOI: 10.1016/j.jff.2018.05.047 Karin G.M. Lenssen , Aalt Bast , Alie de Boer
|
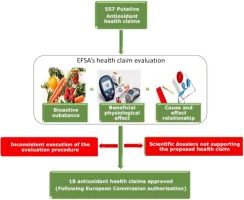
The rejection of many putative health claims in Europe is assumed to have negatively affected functional food innovation. This study analysed the influence of Article 13.1 health claims review procedure on the perception of functional food innovation.
The analysis of all scientific opinions related to antioxidants and a subsequent qualitative review of five scientific dossiers reveals that the evaluation by the European Food Safety Authority was not conducted consistently, as the Authority did not follow their own procedure. Several submitted scientific dossiers however also contain studies unrelated to the proposed health claim.
By not following their own procedure, the European Food Safety Authority has created uncertainty regarding health claim evaluations. This uncertainty presents a risk for food companies and impacts future investments for research and development of new functional food products. Although published guidance documents have partially clarified the evaluation process, further standardisation and clarification will benefit functional food innovation.
中文翻译:

阐明EFSA的健康声明评估程序将有益于功能性食品创新
在欧洲,许多假定的健康主张遭到拒绝被认为对功能性食品创新产生了负面影响。这项研究分析了第13.1条健康声明审查程序对功能性食品创新的看法的影响。
对所有与抗氧化剂有关的科学观点的分析以及随后对五份科学文献的定性审查表明,由于食品安全局未遵循自己的程序,因此欧洲食品安全局的评估并非始终如一。但是,一些提交的科学档案也包含与拟议中的健康声明无关的研究。
通过不遵循自己的程序,欧洲食品安全局在健康声明评估方面造成了不确定性。这种不确定性给食品公司带来了风险,并影响了新功能食品研发方面的未来投资。尽管已发布的指导文件已部分澄清了评估过程,但进一步的标准化和澄清将有益于功能性食品创新。











































 京公网安备 11010802027423号
京公网安备 11010802027423号