当前位置:
X-MOL 学术
›
Catal. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Laccase-catalyzed in situ generation and regeneration of N-phenyltriazolinedione for the aerobic oxidative homo-coupling of thiols to disulfides
Catalysis Communications ( IF 3.4 ) Pub Date : 2018-06-09 , DOI: 10.1016/j.catcom.2018.06.007 Donya Khaledian , Amin Rostami , Seyed Amir Zarei
中文翻译:

漆酶催化原位生成和再生N-苯基三唑啉二酮,用于硫醇与二硫化物的好氧氧化均相偶联
更新日期:2018-06-09
Catalysis Communications ( IF 3.4 ) Pub Date : 2018-06-09 , DOI: 10.1016/j.catcom.2018.06.007 Donya Khaledian , Amin Rostami , Seyed Amir Zarei
|
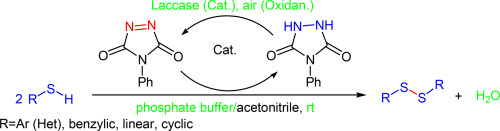
The first report on aerobic in situ generation and regeneration of N-phenyltriazolinedione, a valuable oxidizing agent, from a catalytic amount of N-phenyl urazole in the presence of a laccase enzyme is presented. The application of a 4-phenyl urazole/Laccase/O2 as a new cooperative catalytic oxidation system is reported for a transition-metal-free and halogen free oxidative homo-coupling reaction of structurally diverse thiols to their corresponding disulfides with good to excellent yields in a phosphate buffer solution under mild reaction conditions.
中文翻译:

漆酶催化原位生成和再生N-苯基三唑啉二酮,用于硫醇与二硫化物的好氧氧化均相偶联
有氧的第一份报告原位生成和再生Ñ -phenyltriazolinedione,一种有价值的氧化剂,从催化量ñ -苯基尿唑在漆酶的存在下被呈现。据报道,使用4-苯基脲唑/漆酶/ O 2作为新的协同催化氧化系统,可以使结构多样的硫醇与相应的二硫键发生无过渡金属和无卤素的氧化均偶联反应,并具有良好或优异的收率。在温和的反应条件下在磷酸盐缓冲液中溶解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号