当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Biochemical Basis of Vitamin A Production from the Asymmetric Carotenoid β-Cryptoxanthin
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-06-08 00:00:00 , DOI: 10.1021/acschembio.8b00290 Mary E Kelly 1 , Srinivasagan Ramkumar 1 , Weizhong Sun 1 , Crystal Colon Ortiz 1 , Philip D Kiser 1 , Marcin Golczak 1 , Johannes von Lintig 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-06-08 00:00:00 , DOI: 10.1021/acschembio.8b00290 Mary E Kelly 1 , Srinivasagan Ramkumar 1 , Weizhong Sun 1 , Crystal Colon Ortiz 1 , Philip D Kiser 1 , Marcin Golczak 1 , Johannes von Lintig 1
Affiliation
|
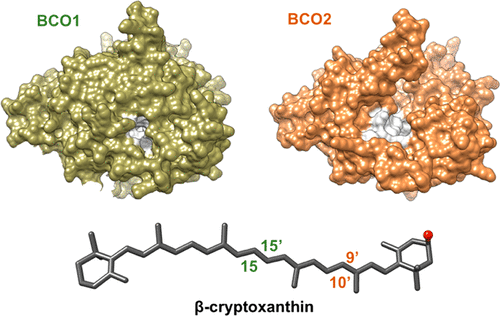
Vitamin A serves essential functions in mammalian biology as a signaling molecule and chromophore. This lipid can be synthesized from more than 50 putative dietary provitamin A precursor molecules which contain at least one unsubstituted β-ionone ring. We here scrutinized the enzymatic properties and substrate specificities of the two structurally related carotenoid cleavage dioxygenases (CCDs) which catalyze this synthesis. Recombinant BCO1 split substrates across the C15,C15′ double bond adjacent to a canonical β-ionone ring site to vitamin A aldehyde. Substitution of the ring with a hydroxyl group prevented this conversion. The removal of methyl groups from the polyene carbon backbone of the substrate did not impede enzyme activity. Homology modeling and site-directed mutagenesis identified amino acid residues at the entrance of the substrate tunnel, which determined BCO1’s specificity for the canonical β-ionone ring site. In contrast, BCO2 split substrates across the C9,C10 double bond adjacent to assorted ionone ring sites. Kinetic analysis revealed a higher catalytic efficiency of BCO2 with substrates bearing 3-hydroxy-β-ionone rings. In the mouse intestine, the asymmetric carotenoid β-cryptoxanthin with one canonical and one 3-hydroxy-β-ionone ring site was meticulously converted to vitamin A. The tailoring of this asymmetric substrate occurred by a stepwise processing of the carotenoid substrate by both CCDs and involved a β-apo-10′-carotenal intermediate. Thus, opposite selectivity for ionone ring sites of the two mammalian CCDs complement each other in the metabolic challenge of vitamin A production from a chemically diverse set of precursor molecules.
中文翻译:

从不对称类胡萝卜素 β-隐黄质生产维生素 A 的生化基础
维生素 A 作为信号分子和发色团在哺乳动物生物学中发挥着重要作用。这种脂质可以由超过 50 种推定的膳食维生素原 A 前体分子合成,其中含有至少一个未取代的 β-紫罗兰酮环。我们在这里仔细研究了催化这种合成的两种结构相关的类胡萝卜素裂解双加氧酶(CCD)的酶性质和底物特异性。重组 BCO1 将底物穿过与典型 β-紫罗兰酮环位点相邻的 C15,C15' 双键裂解为维生素 A 醛。用羟基取代环阻止了这种转化。从底物的多烯碳主链上除去甲基并不会妨碍酶活性。同源建模和定点诱变鉴定了底物通道入口处的氨基酸残基,这确定了 BCO1 对典型 β-紫罗兰酮环位点的特异性。相比之下,BCO2 穿过邻近各种紫罗兰酮环位点的 C9、C10 双键分裂底物。动力学分析表明,带有 3-羟基-β-紫罗兰酮环的底物 BCO2 具有更高的催化效率。在小鼠肠道中,具有一个典型环位点和一个 3-羟基-β-紫罗兰酮环位点的不对称类胡萝卜素 β-隐黄质被精心转化为维生素 A。这种不对称底物的定制是通过两个 CCD 对类胡萝卜素底物的逐步处理而实现的并涉及β-apo-10'-胡萝卜素中间体。因此,两种哺乳动物 CCD 对紫罗兰酮环位点的相反选择性在从一组化学上不同的前体分子生产维生素 A 的代谢挑战中相互补充。
更新日期:2018-06-08
中文翻译:

从不对称类胡萝卜素 β-隐黄质生产维生素 A 的生化基础
维生素 A 作为信号分子和发色团在哺乳动物生物学中发挥着重要作用。这种脂质可以由超过 50 种推定的膳食维生素原 A 前体分子合成,其中含有至少一个未取代的 β-紫罗兰酮环。我们在这里仔细研究了催化这种合成的两种结构相关的类胡萝卜素裂解双加氧酶(CCD)的酶性质和底物特异性。重组 BCO1 将底物穿过与典型 β-紫罗兰酮环位点相邻的 C15,C15' 双键裂解为维生素 A 醛。用羟基取代环阻止了这种转化。从底物的多烯碳主链上除去甲基并不会妨碍酶活性。同源建模和定点诱变鉴定了底物通道入口处的氨基酸残基,这确定了 BCO1 对典型 β-紫罗兰酮环位点的特异性。相比之下,BCO2 穿过邻近各种紫罗兰酮环位点的 C9、C10 双键分裂底物。动力学分析表明,带有 3-羟基-β-紫罗兰酮环的底物 BCO2 具有更高的催化效率。在小鼠肠道中,具有一个典型环位点和一个 3-羟基-β-紫罗兰酮环位点的不对称类胡萝卜素 β-隐黄质被精心转化为维生素 A。这种不对称底物的定制是通过两个 CCD 对类胡萝卜素底物的逐步处理而实现的并涉及β-apo-10'-胡萝卜素中间体。因此,两种哺乳动物 CCD 对紫罗兰酮环位点的相反选择性在从一组化学上不同的前体分子生产维生素 A 的代谢挑战中相互补充。











































 京公网安备 11010802027423号
京公网安备 11010802027423号