当前位置:
X-MOL 学术
›
Environ. Sci. Technol. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Different Mechanisms of Alkaline and Enzymatic Hydrolysis of the Insensitive Munition Component 2,4-Dinitroanisole Lead to Identical Products
Environmental Science & Technology Letters ( IF 8.9 ) Pub Date : 2018-06-08 , DOI: 10.1021/acs.estlett.8b00258 Bridget A. Ulrich 1 , Mallory Palatucci 2 , Jakov Bolotin 1 , Jim C. Spain 2 , Thomas B. Hofstetter 1, 3
Environmental Science & Technology Letters ( IF 8.9 ) Pub Date : 2018-06-08 , DOI: 10.1021/acs.estlett.8b00258 Bridget A. Ulrich 1 , Mallory Palatucci 2 , Jakov Bolotin 1 , Jim C. Spain 2 , Thomas B. Hofstetter 1, 3
Affiliation
|
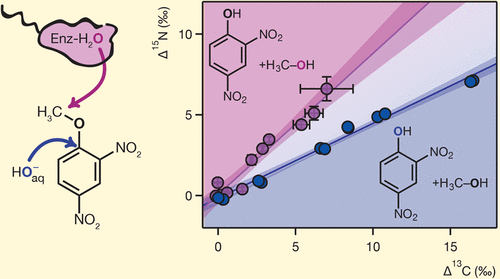
The emerging use of 2,4-dinitroanisole (DNAN) in insensitive munitions formulations has caused concern about future contamination of subsurface environments, generating significant interest in understanding and identifying its transformation processes. Here we characterized the C and N isotope fractionation associated with abiotic and biological DNAN hydrolysis through alkaline hydrolysis at high pH as well as enzymatic hydrolysis by Nocardioides sp. JS1661 and partially purified DNAN O-demethylase. Whereas both reactions generated 2,4-dinitrophenol (DNP), compound-specific isotope analysis (CSIA) of DNAN and DNP revealed that these reactions occur by different mechanisms. Alkaline hydrolysis was associated with apparent 13C and 15N kinetic isotope effects (13C-AKIE and 15N-AKIE) of 1.044 ± 0.003 and 1.0027 ± 0.0004, respectively, reflecting the previously postulated nucleophilic aromatic substitution mechanism. Conversely, enzyme-catalyzed DNAN hydrolysis exhibited a 13C-AKIE of 1.027 ± 0.005 and a 15N-AKIE of 1.0032 ± 0.0003. On the basis of these AKIE values and the C and N isotope fractionation of DNP, our results imply that enzymatic O-demethylation of DNAN occurs through a nucleophilic substitution reaction at the aliphatic C of the methoxy group. This work provides a basis for the assessment of DNAN transformation by CSIA, as the C and N isotope fractionation patterns observed in this work are distinct from other hypothesized degradation pathways.
中文翻译:

碱和酶水解不敏感的弹药成分2,4-二硝基苯甲醚导致相同产物的不同机理
2,4-二硝基苯甲醚(DNAN)在不敏感的弹药配方中的新兴使用引起了人们对地下环境未来污染的担忧,引起了人们对理解和鉴定其转化过程的浓厚兴趣。在这里,我们表征非生物和生物DNAN水解通过碱水解在高pH以及通过酶水解相关联的C和N的同位素分馏类诺卡氏菌。JS1661和部分纯化的DNAN O-脱甲基酶。尽管两个反应均生成2,4-二硝基苯酚(DNP),但DNAN和DNP的化合物特异性同位素分析(CSIA)表明这些反应是通过不同的机理发生的。碱性水解与表观13 C和15有关N动力学同位素效应(13 C-AKIE和15 N-AKIE)分别为1.044±0.003和1.0027±0.0004,反映了先前假定的亲核芳香族取代机理。相反,酶催化的DNAN水解显示出1.027±0.005的13 C-AKIE和1.0032±0.0003的15 N-AKIE。根据这些AKIE值和DNP的C和N同位素分馏,我们的结果表明酶ODNAN的去甲基化是通过甲氧基的脂族C上的亲核取代反应发生的。这项工作为评估CSIA转化DNAN提供了基础,因为在这项工作中观察到的C和N同位素分馏模式与其他假设的降解途径不同。
更新日期:2018-06-09
中文翻译:

碱和酶水解不敏感的弹药成分2,4-二硝基苯甲醚导致相同产物的不同机理
2,4-二硝基苯甲醚(DNAN)在不敏感的弹药配方中的新兴使用引起了人们对地下环境未来污染的担忧,引起了人们对理解和鉴定其转化过程的浓厚兴趣。在这里,我们表征非生物和生物DNAN水解通过碱水解在高pH以及通过酶水解相关联的C和N的同位素分馏类诺卡氏菌。JS1661和部分纯化的DNAN O-脱甲基酶。尽管两个反应均生成2,4-二硝基苯酚(DNP),但DNAN和DNP的化合物特异性同位素分析(CSIA)表明这些反应是通过不同的机理发生的。碱性水解与表观13 C和15有关N动力学同位素效应(13 C-AKIE和15 N-AKIE)分别为1.044±0.003和1.0027±0.0004,反映了先前假定的亲核芳香族取代机理。相反,酶催化的DNAN水解显示出1.027±0.005的13 C-AKIE和1.0032±0.0003的15 N-AKIE。根据这些AKIE值和DNP的C和N同位素分馏,我们的结果表明酶ODNAN的去甲基化是通过甲氧基的脂族C上的亲核取代反应发生的。这项工作为评估CSIA转化DNAN提供了基础,因为在这项工作中观察到的C和N同位素分馏模式与其他假设的降解途径不同。











































 京公网安备 11010802027423号
京公网安备 11010802027423号