当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multiphase Mechanism for the Production of Sulfuric Acid from SO2 by Criegee Intermediates Formed During the Heterogeneous Reaction of Ozone with Squalene
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2018-06-08 00:00:00 , DOI: 10.1021/acs.jpclett.8b01171 Nadja Heine 1 , Caleb Arata 2, 3 , Allen H. Goldstein 3 , Frances A. Houle 1 , Kevin R. Wilson 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2018-06-08 00:00:00 , DOI: 10.1021/acs.jpclett.8b01171 Nadja Heine 1 , Caleb Arata 2, 3 , Allen H. Goldstein 3 , Frances A. Houle 1 , Kevin R. Wilson 1
Affiliation
|
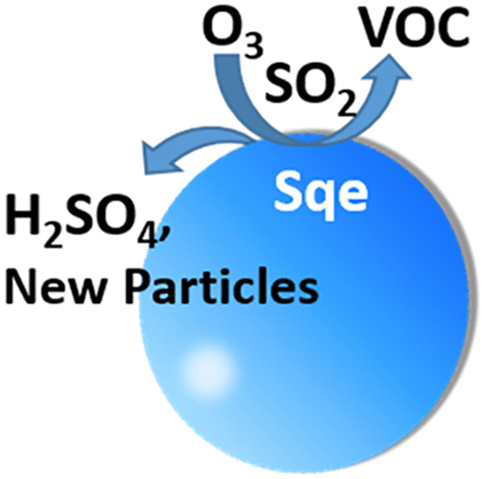
Here we report a new multiphase reaction mechanism by which Criegee intermediates (CIs), formed by ozone reactions at an alkene surface, convert SO2 to SO3 to produce sulfuric acid, a precursor for new particle formation (NPF). During the heterogeneous ozone reaction, in the presence of 220 ppb SO2, an unsaturated aerosol (squalene) undergoes rapid chemical erosion, which is accompanied by NPF. A kinetic model predicts that the mechanism for chemical erosion and NPF originate from a common elementary step (CI + SO2) that produces both gas phase SO3 and small ketones. At low relative humidity (RH = 5%), 20% of the aerosol mass is lost, with 17% of the ozone-surface reactions producing SO3. At RH = 60%, the aerosol shrinks by 30%, and the yield of SO3 is <5%. This multiphase formation mechanism of H2SO4 by CIs is discussed in the context of indoor air quality and atmospheric chemistry.
中文翻译:

臭氧与角鲨烯异相反应过程中形成的Criegee中间体由SO 2生产硫酸的多相机理
在这里,我们报告了一种新的多相反应机理,通过该机理,烯烃表面上的臭氧反应形成的Criegee中间体(CIs)将SO 2转化为SO 3以产生硫酸,硫酸是新颗粒形成(NPF)的前体。在非均相臭氧反应过程中,在220 ppb SO 2的存在下,不饱和气溶胶(角鲨烯)经历了快速的化学腐蚀,并伴有NPF。动力学模型预测化学腐蚀和NPF的机理源自共同的基本步骤(CI + SO 2),该步骤同时生成气相SO 3和小酮。在较低的相对湿度(RH = 5%)下,会损失20%的气溶胶质量,其中17%的臭氧表面反应会产生SO 3。在RH = 60%时,气溶胶收缩30%,SO 3的产率<5%。在室内空气质量和大气化学的背景下,讨论了CIs形成H 2 SO 4的这种多相形成机理。
更新日期:2018-06-08
中文翻译:

臭氧与角鲨烯异相反应过程中形成的Criegee中间体由SO 2生产硫酸的多相机理
在这里,我们报告了一种新的多相反应机理,通过该机理,烯烃表面上的臭氧反应形成的Criegee中间体(CIs)将SO 2转化为SO 3以产生硫酸,硫酸是新颗粒形成(NPF)的前体。在非均相臭氧反应过程中,在220 ppb SO 2的存在下,不饱和气溶胶(角鲨烯)经历了快速的化学腐蚀,并伴有NPF。动力学模型预测化学腐蚀和NPF的机理源自共同的基本步骤(CI + SO 2),该步骤同时生成气相SO 3和小酮。在较低的相对湿度(RH = 5%)下,会损失20%的气溶胶质量,其中17%的臭氧表面反应会产生SO 3。在RH = 60%时,气溶胶收缩30%,SO 3的产率<5%。在室内空气质量和大气化学的背景下,讨论了CIs形成H 2 SO 4的这种多相形成机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号