当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Zn(OTf)2 catalyzed Ugi-type reaction of 3-(2-isocyanoethyl)indoles with indole-derived ketimines: rapid access to hexacyclic spiroindolines†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-06-08 00:00:00 , DOI: 10.1039/c8qo00382c Yun-Lin Liu 1, 2, 3, 4 , Xiang-Yu Mao 1, 2, 3, 4 , Xiao-Tong Lin 1, 2, 3, 4 , Guo-Shu Chen 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-06-08 00:00:00 , DOI: 10.1039/c8qo00382c Yun-Lin Liu 1, 2, 3, 4 , Xiang-Yu Mao 1, 2, 3, 4 , Xiao-Tong Lin 1, 2, 3, 4 , Guo-Shu Chen 1, 2, 3, 4
Affiliation
|
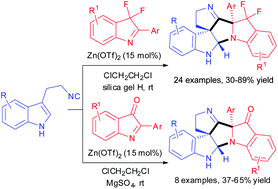
We report a Zn(OTf)2 catalyzed Ugi-type reaction of 3-(2-isocyanoethyl)indoles and indole-derived ketimines to rapidly afford hexacyclic spiroindolines featuring three stereocenters including two quaternary stereocenters in moderate to excellent yields (30–89%) with complete diastereoselectivity. This reaction is highly efficient because two C–C and one C–N bonds as well as two new rings are created under mild reaction conditions in a single step.
中文翻译:

Zn(OTf)2催化3-(2-异氰乙基)吲哚与吲哚衍生的酮亚胺的Ugi型反应:快速获得六环螺二吲哚†
我们报道了Zn(OTf)2催化的3-(2-异氰乙基)吲哚和吲哚衍生的酮亚胺的Ugi型反应,以迅速提供具有三个立体中心的六环螺二氢吲哚,包括两个中等至极好的立体中心(四元立体中心)(30-89%)具有完全的非对映选择性。该反应非常有效,因为在温和的反应条件下,只需一步即可生成两个C–C和一个C–N键以及两个新的环。
更新日期:2018-06-08
中文翻译:

Zn(OTf)2催化3-(2-异氰乙基)吲哚与吲哚衍生的酮亚胺的Ugi型反应:快速获得六环螺二吲哚†
我们报道了Zn(OTf)2催化的3-(2-异氰乙基)吲哚和吲哚衍生的酮亚胺的Ugi型反应,以迅速提供具有三个立体中心的六环螺二氢吲哚,包括两个中等至极好的立体中心(四元立体中心)(30-89%)具有完全的非对映选择性。该反应非常有效,因为在温和的反应条件下,只需一步即可生成两个C–C和一个C–N键以及两个新的环。










































 京公网安备 11010802027423号
京公网安备 11010802027423号