当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lactamization of sp2 C–H bonds with CO2 under transition-metal-free and redox-neutral conditions: a computational mechanistic study†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-06-08 00:00:00 , DOI: 10.1039/c8qo00394g Weiyi Li 1, 2, 3, 4 , Caiqin Li 5, 6, 7, 8 , Yajing Lyu 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-06-08 00:00:00 , DOI: 10.1039/c8qo00394g Weiyi Li 1, 2, 3, 4 , Caiqin Li 5, 6, 7, 8 , Yajing Lyu 1, 2, 3, 4
Affiliation
|
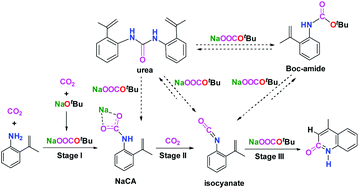
The detailed mechanism for the lactamization of sp2 C–H bonds in aniline with CO2 to 2-quinoline under transition-metal-free and redox-neutral conditions was studied by density functional theory (DFT) calculations. Among all the examined reaction pathways over the base, we found that the minimum energy reaction pathway (MERP) consists of three stages: (i) the reaction of aniline and CO2 to generate the sodium salt of carbamate (NaCA), (ii) the deoxygenation of the NaCA species to form the isocyanate intermediate, and (iii) the intramolecular cyclization of the isocyanate group to the sp2 C–H bond. Overall, the deoxygenation of the NaCA species in stage (ii) is rate-determining for the entire reaction. The base plays a significant role in reducing the energy barriers for the formation of the NaCA species and the intramolecular cyclization of the isocyanate group to the sp2 C–H bond. The free CO2 serves as the atomic oxidant [O] acceptor in the concept of ‘CO2 = CO + O’ proposed for the carbonylation reaction. In addition, the calculations disclose that the intermolecular addition of the tert-butanol (HOtBu) intermediate or substrate aniline to the isocyanate intermediate is kinetically competitive with the intramolecular electrophilic cyclization of the isocyanate group to the sp2 C–H bond, which successfully accounts for the experimental detection of the tert-butyloxy carbonyl (Boc) protected amide (Boc-amide) and urea intermediates in the reaction mixture. As the base cannot be regenerated along with the formation of the final product, it is predicted that weak basic species like Na2CO3 or NaHCO3 in the reaction system might be effective in promoting the reaction as well. This finding provides a rational interpretation of the experimental observation that the excess of base and high temperature are necessary for the high efficiency of the reaction.
中文翻译:

在无过渡金属和氧化还原中性条件下 ,sp 2 C–H键与CO 2的内酰胺化:计算机制研究†
通过密度泛函理论(DFT)计算研究了在无过渡金属和氧化还原中性条件下,苯胺中的sp 2 C–H键与CO 2转化为2-喹啉内酰胺化的详细机理。在所有经过检查的碱反应途径中,我们发现最小能量反应途径(MERP)包括三个阶段:(i)苯胺和CO 2反应生成氨基甲酸钠(NaCA),(ii) NaCA物种的脱氧以形成异氰酸酯中间体,以及(iii)异氰酸酯基团的分子内环化成sp 2碳氢键。总体而言,阶段(ii)中NaCA物质的脱氧决定了整个反应的速率。该碱在减少形成NaCA物种的能垒和异氰酸酯基团到sp 2 C–H键的分子内环化方面起着重要作用。在为羰基化反应提出的“ CO 2= CO + O”的概念中,游离的CO 2用作原子氧化剂[O]受体。另外,该计算公开了叔丁醇(HO t Bu)中间体或底物苯胺在异氰酸酯中间体中的分子间加成与异氰酸酯基团对sp的分子内亲电环化具有动力学竞争性。2个C–H键,成功地说明了在反应混合物中对叔丁氧羰基(Boc)保护的酰胺(Boc-酰胺)和尿素中间体的实验检测。由于碱不能随着最终产物的形成而再生,因此可以预测,反应体系中的弱碱性物质(如Na 2 CO 3或NaHCO 3)也可能有效地促进了反应。这一发现为实验观察提供了合理的解释,即碱的过量和高温对于反应的高效率是必要的。
更新日期:2018-06-08
中文翻译:

在无过渡金属和氧化还原中性条件下 ,sp 2 C–H键与CO 2的内酰胺化:计算机制研究†
通过密度泛函理论(DFT)计算研究了在无过渡金属和氧化还原中性条件下,苯胺中的sp 2 C–H键与CO 2转化为2-喹啉内酰胺化的详细机理。在所有经过检查的碱反应途径中,我们发现最小能量反应途径(MERP)包括三个阶段:(i)苯胺和CO 2反应生成氨基甲酸钠(NaCA),(ii) NaCA物种的脱氧以形成异氰酸酯中间体,以及(iii)异氰酸酯基团的分子内环化成sp 2碳氢键。总体而言,阶段(ii)中NaCA物质的脱氧决定了整个反应的速率。该碱在减少形成NaCA物种的能垒和异氰酸酯基团到sp 2 C–H键的分子内环化方面起着重要作用。在为羰基化反应提出的“ CO 2= CO + O”的概念中,游离的CO 2用作原子氧化剂[O]受体。另外,该计算公开了叔丁醇(HO t Bu)中间体或底物苯胺在异氰酸酯中间体中的分子间加成与异氰酸酯基团对sp的分子内亲电环化具有动力学竞争性。2个C–H键,成功地说明了在反应混合物中对叔丁氧羰基(Boc)保护的酰胺(Boc-酰胺)和尿素中间体的实验检测。由于碱不能随着最终产物的形成而再生,因此可以预测,反应体系中的弱碱性物质(如Na 2 CO 3或NaHCO 3)也可能有效地促进了反应。这一发现为实验观察提供了合理的解释,即碱的过量和高温对于反应的高效率是必要的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号