当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pd(ii)-Catalyzed gamma-C(sp3)–H alkynylation of amides: selective functionalization of R chains of amides R1C(O)NHR†
Chemical Communications ( IF 4.3 ) Pub Date : 2018-06-08 00:00:00 , DOI: 10.1039/c8cc03445a Vinod G. Landge 1, 2, 3, 4 , Ayisha Parveen 1, 2, 3, 4 , Avanashiappan Nandakumar 1, 2, 3, 4 , Ekambaram Balaraman 1, 2, 3, 4
Chemical Communications ( IF 4.3 ) Pub Date : 2018-06-08 00:00:00 , DOI: 10.1039/c8cc03445a Vinod G. Landge 1, 2, 3, 4 , Ayisha Parveen 1, 2, 3, 4 , Avanashiappan Nandakumar 1, 2, 3, 4 , Ekambaram Balaraman 1, 2, 3, 4
Affiliation
|
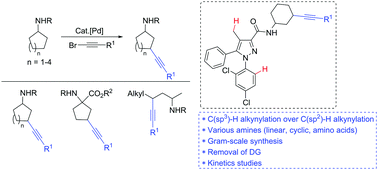
The gamma C(sp3)–H bond alkynylation of R chains of amides R1C(O)NHR, a fundamental class of synthetic substrates, has not been accomplished to date. Here, the first example of palladium(II)-catalyzed alkynylation of an unactivated gamma C(sp3)–H bond of alkyl amides (cyclic, linear, and amino acids) is reported. The kinetic experiment shows that the rate of the reaction depends on the coupling partners and the amides. Late-stage diversification of alkynylated amides was developed by utilizing amine and alkyne functionalities.
中文翻译:

Pd(ii)催化的酰胺的γ-C(sp 3)-H烷基化:酰胺R链的选择性官能化R 1 C(O)NHR †
到目前为止,尚未完成酰胺R 1 C(O)NHR的R链的C链C(sp 3)-H键的炔基化反应。在此,报道了钯(II)催化烷基酰胺(环状,线性和氨基酸)的未活化γC(sp 3)-H键的炔基化反应的第一个例子。动力学实验表明,反应速率取决于偶联伙伴和酰胺。通过利用胺和炔烃官能团开发了烷基化酰胺的后期多样化产品。
更新日期:2018-06-08
中文翻译:

Pd(ii)催化的酰胺的γ-C(sp 3)-H烷基化:酰胺R链的选择性官能化R 1 C(O)NHR †
到目前为止,尚未完成酰胺R 1 C(O)NHR的R链的C链C(sp 3)-H键的炔基化反应。在此,报道了钯(II)催化烷基酰胺(环状,线性和氨基酸)的未活化γC(sp 3)-H键的炔基化反应的第一个例子。动力学实验表明,反应速率取决于偶联伙伴和酰胺。通过利用胺和炔烃官能团开发了烷基化酰胺的后期多样化产品。











































 京公网安备 11010802027423号
京公网安备 11010802027423号