当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solid–Liquid Phase Equilibria of the Ternary System (2-Naphthaldehyde + 4-Methylphthalic Anhydride + Ethyl Acetate) at (288.15, 298.15, and 308.15) K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-06-08 , DOI: 10.1021/acs.jced.8b00170 Xiaoqiang Gao 1 , Fang Zhang 2 , Yi Yu 1 , Yonghui Dou 1 , Li Xu 1 , Guoji Liu 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-06-08 , DOI: 10.1021/acs.jced.8b00170 Xiaoqiang Gao 1 , Fang Zhang 2 , Yi Yu 1 , Yonghui Dou 1 , Li Xu 1 , Guoji Liu 1
Affiliation
|
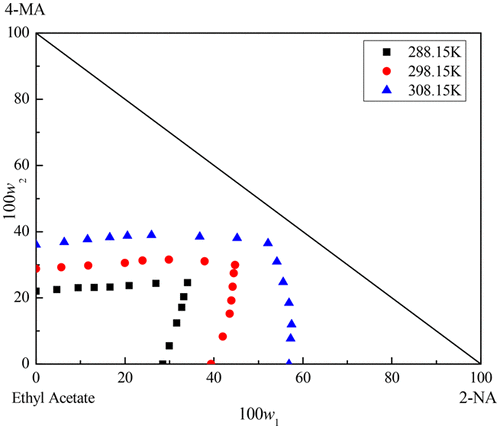
The solubility of 2-naphthaldehyde (2-NA) and 4-methylphthalic anhydride (4-MA) in ethyl acetate at temperatures from 283.15 to 313.15 K and solid–liquid equilibrium of ternary (2-NA + 4-MA + ethyl acetate) system at temperatures of (288.15, 298.15, and 308.15) K were measured under pressure of 101.3 kPa. Three isothermal phase diagrams were plotted according to the ternary solubility data. For the ternary system, two pure 2-NA and 4-MA solids were formed at each temperature and determined by the wet residue method of Schreinemaker. The crystalline region of 2-NA decreases with the increase in temperature, and 4-MA is just the opposite. The crystallization region of 2-NA was found to be smaller than that of 4-MA. The solubility data of the system were correlated and calculated by NRTL and Wilson models. The results show that the NRTL model agrees well with the experimental data compared with the Wilson model. Furthermore, the densities of equilibrium solutions were determined at the corresponding temperatures.
中文翻译:

三元体系(2-萘醛+ 4-甲基邻苯二甲酸酐+乙酸乙酯)的固-液相平衡(288.15、298.15和308.15)K
在283.15至313.15 K的温度下,2-萘醛(2-NA)和4-甲基邻苯二甲酸酐(4-MA)在乙酸乙酯中的溶解度以及三元溶液(2-NA + 4-MA +乙酸乙酯)的固液平衡在101.3 kPa的压力下测量了系统在(288.15、298.15和308.15)K的温度下的温度。根据三元溶解度数据绘制了三个等温相图。对于三元体系,在每个温度下均生成两个纯净的2-NA和4-MA固体,并通过Schreinemaker的湿残留法进行测定。2-NA的结晶区域随温度的升高而减小,而4-MA恰好相反。发现2-NA的结晶区域小于4-MA的结晶区域。通过NRTL和Wilson模型对系统的溶解度数据进行关联和计算。结果表明,与Wilson模型相比,NRTL模型与实验数据吻合良好。此外,在相应的温度下确定平衡溶液的密度。
更新日期:2018-06-08
中文翻译:

三元体系(2-萘醛+ 4-甲基邻苯二甲酸酐+乙酸乙酯)的固-液相平衡(288.15、298.15和308.15)K
在283.15至313.15 K的温度下,2-萘醛(2-NA)和4-甲基邻苯二甲酸酐(4-MA)在乙酸乙酯中的溶解度以及三元溶液(2-NA + 4-MA +乙酸乙酯)的固液平衡在101.3 kPa的压力下测量了系统在(288.15、298.15和308.15)K的温度下的温度。根据三元溶解度数据绘制了三个等温相图。对于三元体系,在每个温度下均生成两个纯净的2-NA和4-MA固体,并通过Schreinemaker的湿残留法进行测定。2-NA的结晶区域随温度的升高而减小,而4-MA恰好相反。发现2-NA的结晶区域小于4-MA的结晶区域。通过NRTL和Wilson模型对系统的溶解度数据进行关联和计算。结果表明,与Wilson模型相比,NRTL模型与实验数据吻合良好。此外,在相应的温度下确定平衡溶液的密度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号