当前位置:
X-MOL 学术
›
Food Funct.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impacts of glycation and transglutaminase-catalyzed glycosylation with glucosamine on the conformational structure and allergenicity of bovine β-lactoglobulin
Food & Function ( IF 5.1 ) Pub Date : 2018-06-08 00:00:00 , DOI: 10.1039/c8fo00909k Fangzhou Yuan 1, 2, 3, 4, 5 , Ishfaq Ahmed 6, 7, 8, 9 , Liangtao Lv 6, 7, 8, 9 , Zhaojie Li 9, 10, 11 , Zhenxing Li 6, 7, 8, 9 , Hong Lin 6, 7, 8, 9 , Hang Lin 8, 9, 12 , Jinxia Zhao 8, 9, 12 , Shenglan Tian 6, 7, 8, 9 , Jiaju Ma 6, 7, 8, 9
Food & Function ( IF 5.1 ) Pub Date : 2018-06-08 00:00:00 , DOI: 10.1039/c8fo00909k Fangzhou Yuan 1, 2, 3, 4, 5 , Ishfaq Ahmed 6, 7, 8, 9 , Liangtao Lv 6, 7, 8, 9 , Zhaojie Li 9, 10, 11 , Zhenxing Li 6, 7, 8, 9 , Hong Lin 6, 7, 8, 9 , Hang Lin 8, 9, 12 , Jinxia Zhao 8, 9, 12 , Shenglan Tian 6, 7, 8, 9 , Jiaju Ma 6, 7, 8, 9
Affiliation
|
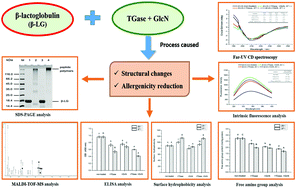
β-Lactoglobulin (β-LG) is recognized as the major milk allergen. In this study, the effects of transglutaminase (TGase) and glucosamine (GlcN)-catalyzed glycosylation and glycation on the conformational structure and allergenicity of β-LG were investigated. The formations of cross-linked peptides were demonstrated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and GlcN-conjugated modification was identified using matrix-assisted laser desorption ionization-time of flight-mass spectrometry (MALDI-TOF-MS). Structural analysis revealed that glycosylation and glycation of β-LG induced unfolding of the primary protein structure followed by a loss of the secondary structure. As revealed by circular dichroism (CD) spectroscopy, glycosylated β-LG exhibited the highest increase in the β-sheets from 32.6% to 40.4% (25 °C) and 44.2% (37 °C), and the percentage of α-helices decreased from 17.7% to 14.4% (25 °C) and 12.3% (37 °C), respectively. The tertiary and quaternary structures of β-LG also changed significantly during glycosylation and glycation, along with reduced free amino groups and variation in surface hydrophobicity. Immunoblotting and indirect enzyme-linked immuno sorbent assay (ELISA) analyses demonstrated that the lowest IgG- and IgE-binding capacities of β-LG were obtained following glycosylation at 37 °C, which were 52.7% and 56.3% lower than that of the native protein, respectively. The reduction in the antigenicity and potential allergenicity of glycosylated β-LG was more pronounced compared to TGase treated- and glycated β-LG, which correlated well with the structural changes. These results suggest that TGase-catalyzed glycosylation has more potential compared to glycation for mitigating the allergenic potential of milk products.
中文翻译:

氨基葡萄糖糖基化和转谷氨酰胺酶催化的糖基化对牛β-乳球蛋白构象结构和变应原性的影响
β-乳球蛋白(β-LG)被认为是主要的牛奶过敏原。在这项研究中,研究了转谷氨酰胺酶(TGase)和葡糖胺(GlcN)催化的糖基化和糖基化对β-LG构象结构和变应原性的影响。十二烷基硫酸钠-聚丙烯酰胺凝胶电泳(SDS-PAGE)证明了交联肽的形成,并使用基质辅助激光解吸电离飞行时间质谱(MALDI-TOF-MS)鉴定了GlcN共轭修饰。 )。结构分析表明,β-LG的糖基化和糖基化诱导一级蛋白结构的展开,继而二级结构的损失。如圆二色性(CD)光谱所揭示的,糖基化的β-LG在β片层中的增幅最高,从32.6%增至40.4%(25°C)和44。2%(37°C),α螺旋的百分比分别从17.7%降至14.4%(25°C)和12.3%(37°C)。在糖基化和糖基化过程中,β-LG的三级和四级结构也发生了显着变化,同时游离氨基减少,表面疏水性发生变化。免疫印迹和间接酶联免疫吸附试验(ELISA)分析表明,在37°C糖基化后,β-LG的IgG和IgE结合能力最低,分别比天然抗体低52.7%和56.3%蛋白质。与TGase处理和糖基化的β-LG相比,糖基化的β-LG的抗原性和潜在的变应原性降低更为明显,这与结构变化密切相关。
更新日期:2018-06-08
中文翻译:

氨基葡萄糖糖基化和转谷氨酰胺酶催化的糖基化对牛β-乳球蛋白构象结构和变应原性的影响
β-乳球蛋白(β-LG)被认为是主要的牛奶过敏原。在这项研究中,研究了转谷氨酰胺酶(TGase)和葡糖胺(GlcN)催化的糖基化和糖基化对β-LG构象结构和变应原性的影响。十二烷基硫酸钠-聚丙烯酰胺凝胶电泳(SDS-PAGE)证明了交联肽的形成,并使用基质辅助激光解吸电离飞行时间质谱(MALDI-TOF-MS)鉴定了GlcN共轭修饰。 )。结构分析表明,β-LG的糖基化和糖基化诱导一级蛋白结构的展开,继而二级结构的损失。如圆二色性(CD)光谱所揭示的,糖基化的β-LG在β片层中的增幅最高,从32.6%增至40.4%(25°C)和44。2%(37°C),α螺旋的百分比分别从17.7%降至14.4%(25°C)和12.3%(37°C)。在糖基化和糖基化过程中,β-LG的三级和四级结构也发生了显着变化,同时游离氨基减少,表面疏水性发生变化。免疫印迹和间接酶联免疫吸附试验(ELISA)分析表明,在37°C糖基化后,β-LG的IgG和IgE结合能力最低,分别比天然抗体低52.7%和56.3%蛋白质。与TGase处理和糖基化的β-LG相比,糖基化的β-LG的抗原性和潜在的变应原性降低更为明显,这与结构变化密切相关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号