当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct Genetic and Enzymatic Evidence for Oxidative Cyclization in Hygromycin B Biosynthesis
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-06-07 00:00:00 , DOI: 10.1021/acschembio.8b00375 Sicong Li 1 , Jun Zhang 1 , Yuanzhen Liu 1 , Guo Sun 1 , Zixin Deng 1 , Yuhui Sun 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-06-07 00:00:00 , DOI: 10.1021/acschembio.8b00375 Sicong Li 1 , Jun Zhang 1 , Yuanzhen Liu 1 , Guo Sun 1 , Zixin Deng 1 , Yuhui Sun 1
Affiliation
|
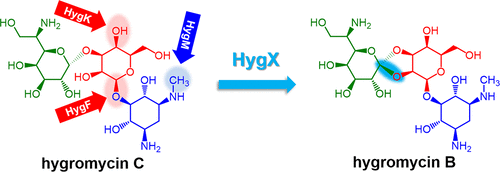
Hygromycin B is an aminoglycoside antibiotic with a structurally distinctive orthoester linkage. Despite its long history of use in industry and in the laboratory, its biosynthesis remains poorly understood. We show here, by in-frame gene deletion in vivo and detailed enzyme characterization in vitro, that formation of the unique orthoester moiety is catalyzed by the α-ketoglutarate- and non-heme iron-dependent oxygenase HygX. In addition, we identify HygF as a glycosyltransferase adding UDP-hexose to 2-deoxystreptamine, HygM as a methyltransferase responsible for N-3 methylation, and HygK as an epimerase. These experimental results and bioinformatic analyses allow a detailed pathway for hygromycin B biosynthesis to be proposed, including the key oxidative cyclization reactions.
中文翻译:

潮霉素B生物合成中氧化环化的直接遗传和酶证据。
潮霉素B是一种氨基糖苷类抗生素,具有结构独特的原酸酯键。尽管其在工业和实验室中的使用已有悠久的历史,但对其生物合成的了解仍然很少。我们在这里通过体内框内基因缺失和体外详细的酶表征显示,独特的原酸酯部分的形成是由α-酮戊二酸和非血红素铁依赖性加氧酶HygX催化的。此外,我们确定HygF为糖基转移酶,将UDP-己糖添加到2-脱氧链胺中,HygM为负责N-3甲基化的甲基转移酶,HygK为差向异构酶。这些实验结果和生物信息学分析允许提出潮霉素B生物合成的详细途径,包括关键的氧化环化反应。
更新日期:2018-06-07
中文翻译:

潮霉素B生物合成中氧化环化的直接遗传和酶证据。
潮霉素B是一种氨基糖苷类抗生素,具有结构独特的原酸酯键。尽管其在工业和实验室中的使用已有悠久的历史,但对其生物合成的了解仍然很少。我们在这里通过体内框内基因缺失和体外详细的酶表征显示,独特的原酸酯部分的形成是由α-酮戊二酸和非血红素铁依赖性加氧酶HygX催化的。此外,我们确定HygF为糖基转移酶,将UDP-己糖添加到2-脱氧链胺中,HygM为负责N-3甲基化的甲基转移酶,HygK为差向异构酶。这些实验结果和生物信息学分析允许提出潮霉素B生物合成的详细途径,包括关键的氧化环化反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号