当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Binding Kinetics of the Intrinsically Disordered p53 Family Transactivation Domains and MDM2
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2018-06-26 , DOI: 10.1021/acs.jpcb.8b03876 Emma Åberg 1 , O. Andreas Karlsson 1 , Eva Andersson 1 , Per Jemth 1
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2018-06-26 , DOI: 10.1021/acs.jpcb.8b03876 Emma Åberg 1 , O. Andreas Karlsson 1 , Eva Andersson 1 , Per Jemth 1
Affiliation
|
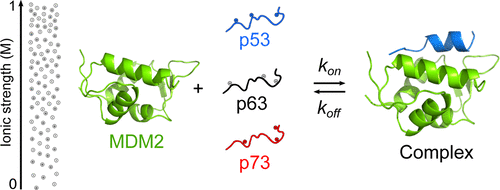
Because of their prominent roles in cell-cycle regulation and cancer, the interaction between MDM2 and the intrinsically disordered transactivation domain (TAD) of p53 is exceptionally well-studied. However, although there are numerous computational studies on the interaction mechanism, there is a paucity of experimental data regarding the kinetics and mechanism. We have used stopped flow fluorescence to investigate the binding reaction between MDM2 and TAD from p53 as well as from its paralogs p63 and p73, and in particular, focused on the salt dependence of the interaction. The observed kinetics are consistent with a two-state mechanism within the time frame of the stopped flow methodology; thus, any conformational changes including the previously identified MDM2 lid dynamics must occur on a time scale <5 ms at 10 °C. The association rate constants are similar for the three TADs, and differences in the dissociation rate constants determine the various affinities with MDM2. In contrast to previous studies, we found a relatively small ionic-strength dependence for all three interactions, highlighting the large variation in the role of electrostatics among binding reactions of intrinsically disordered proteins (IDPs). The basal association rate constants in the absence of electrostatic interactions were relatively high (≥2 × 106 M–1 s–1 at 10 °C), suggesting that a large number of initial contacts may lead to a productive complex. Our findings support an emerging picture of “conformational funneling” occurring in the initial stages of interactions involving IDPs and that these early binding events can rely on hydrophobic as well as charge–charge interactions.
中文翻译:

固有紊乱的p53家族反式激活域和MDM2的结合动力学。
由于它们在细胞周期调控和癌症中的重要作用,因此对MDM2与p53的内在失调的反式激活域(TAD)之间的相互作用进行了很好的研究。然而,尽管有许多关于相互作用机理的计算研究,但是关于动力学和机理的实验数据很少。我们已经使用了停止流动荧光来研究MDM2和TAD从p53以及其旁系同源物p63和p73的结合反应,特别是关注相互作用的盐依赖性。在停止流动方法的时间范围内,观察到的动力学与二态机制相一致。因此,任何构象变化(包括先前确定的MDM2盖子动力学)都必须在10°C的时间范围内小于5 ms。三个TAD的缔合速率常数相似,解离速率常数的差异决定了与MDM2的各种亲和力。与以前的研究相比,我们发现所有三种相互作用的离子强度依赖性都相对较小,这突出说明了固有无序蛋白(IDP)结合反应之间静电作用的巨大差异。在没有静电相互作用的情况下,基础缔合速率常数相对较高(≥2×10 强调了固有无序蛋白(IDP)结合反应之间静电作用的巨大差异。在没有静电相互作用的情况下,基础缔合速率常数相对较高(≥2×10 强调了固有无序蛋白(IDP)结合反应之间静电作用的巨大差异。在没有静电相互作用的情况下,基础缔合速率常数相对较高(≥2×10在10°C时为6 M –1 s –1),这表明大量的初始接触可能会导致生产复杂。我们的发现支持在涉及IDP的相互作用的初始阶段出现“构象漏斗”的新现象,这些早期的结合事件可能依赖于疏水性以及电荷-电荷相互作用。
更新日期:2018-06-27
中文翻译:

固有紊乱的p53家族反式激活域和MDM2的结合动力学。
由于它们在细胞周期调控和癌症中的重要作用,因此对MDM2与p53的内在失调的反式激活域(TAD)之间的相互作用进行了很好的研究。然而,尽管有许多关于相互作用机理的计算研究,但是关于动力学和机理的实验数据很少。我们已经使用了停止流动荧光来研究MDM2和TAD从p53以及其旁系同源物p63和p73的结合反应,特别是关注相互作用的盐依赖性。在停止流动方法的时间范围内,观察到的动力学与二态机制相一致。因此,任何构象变化(包括先前确定的MDM2盖子动力学)都必须在10°C的时间范围内小于5 ms。三个TAD的缔合速率常数相似,解离速率常数的差异决定了与MDM2的各种亲和力。与以前的研究相比,我们发现所有三种相互作用的离子强度依赖性都相对较小,这突出说明了固有无序蛋白(IDP)结合反应之间静电作用的巨大差异。在没有静电相互作用的情况下,基础缔合速率常数相对较高(≥2×10 强调了固有无序蛋白(IDP)结合反应之间静电作用的巨大差异。在没有静电相互作用的情况下,基础缔合速率常数相对较高(≥2×10 强调了固有无序蛋白(IDP)结合反应之间静电作用的巨大差异。在没有静电相互作用的情况下,基础缔合速率常数相对较高(≥2×10在10°C时为6 M –1 s –1),这表明大量的初始接触可能会导致生产复杂。我们的发现支持在涉及IDP的相互作用的初始阶段出现“构象漏斗”的新现象,这些早期的结合事件可能依赖于疏水性以及电荷-电荷相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号