当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enzymatically Activated Glyco-Prodrugs of Doxorubicin Synthesized by a Catalysis-Free Diels–Alder Reaction
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-06-07 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00314 David Bliman 1, 2 , Martine Demeunynck 2 , Pierre Leblond 3, 4 , Samuel Meignan 3, 4 , Isabelle Baussane 2 , Sebastien Fort 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-06-07 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00314 David Bliman 1, 2 , Martine Demeunynck 2 , Pierre Leblond 3, 4 , Samuel Meignan 3, 4 , Isabelle Baussane 2 , Sebastien Fort 1
Affiliation
|
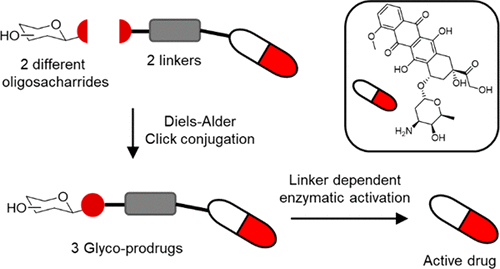
The severe side effects associated with the use of anthracycline anticancer agents continues to limit their use. Herein we describe the synthesis and preliminary biological evaluation of three enzymatically activatable doxorubicin-oligosaccharide prodrugs. The synthetic protocol allows late stage variation of the carbohydrate and is compatible with the use of disaccharides such as lactose as well as more complex oligosaccharides such as xyloglucan oligomers. The enzymatic release of doxorubicin from the prodrugs by both protease (plasmin) and human carboxylesterases (hCE1 and 2) was demonstrated in vitro and the cytotoxic effect of the prodrugs was assayed on MCF-7 breast cancer cells.
中文翻译:

无催化Diels-Alder反应合成的阿霉素酶促糖原药
与蒽环类抗癌药的使用相关的严重副作用继续限制了它们的使用。在这里,我们描述了三种可酶活化的阿霉素-寡糖前药的合成和初步生物学评估。合成方案允许碳水化合物的后期变化,并且与二糖(如乳糖)以及更复杂的低聚糖(如木葡聚糖低聚物)的使用兼容。在体外证明了蛋白酶(纤溶酶)和人羧酸酯酶(hCE1和2)从前药中酶释放阿霉素,并测定了前药对MCF-7乳腺癌细胞的细胞毒性作用。
更新日期:2018-06-07
中文翻译:

无催化Diels-Alder反应合成的阿霉素酶促糖原药
与蒽环类抗癌药的使用相关的严重副作用继续限制了它们的使用。在这里,我们描述了三种可酶活化的阿霉素-寡糖前药的合成和初步生物学评估。合成方案允许碳水化合物的后期变化,并且与二糖(如乳糖)以及更复杂的低聚糖(如木葡聚糖低聚物)的使用兼容。在体外证明了蛋白酶(纤溶酶)和人羧酸酯酶(hCE1和2)从前药中酶释放阿霉素,并测定了前药对MCF-7乳腺癌细胞的细胞毒性作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号