当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cytotoxic and trypanocidal activities of cinchona alkaloid derivatives
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2018-07-01 , DOI: 10.1111/cbdd.13346 Karol Kacprzak 1 , Piotr Ruszkowski 2 , Luisa Valentini 3 , Adam Huczyński 1 , Dietmar Steverding 3
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2018-07-01 , DOI: 10.1111/cbdd.13346 Karol Kacprzak 1 , Piotr Ruszkowski 2 , Luisa Valentini 3 , Adam Huczyński 1 , Dietmar Steverding 3
Affiliation
|
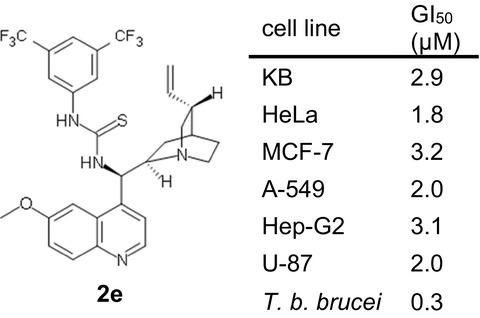
A series of 27 cinchona alkaloid derivatives (1f–w, 2a–e and 3a–d) were investigated for their cytotoxic and trypanocidal activities using seven different cancer cell lines (KB, HeLa, MCF‐7, A‐549, Hep‐G2, U‐87 and HL‐60), two normal cell lines (HDF and CHO) and bloodstream forms of Trypanosoma brucei brucei, respectively. Four compounds (1u, 1w, 2e and 3d) were identified with promising cytotoxic activity with 50% growth inhibition (GI50) values below 10 μM. Two (2e and 3d) of the four compounds also exhibited potent anti‐trypanosomal activity with GI50 values of 0.3–0.4 μM. All four active compounds represented derivatives modified at their C‐9 hydroxy group. With respect to anti‐proliferative activity and selectivity, 2e (epi‐N‐quinidyl‐N′‐bis(3,5‐trifluoromethyl)phenylthiourea) proved to be the most promising derivative for both cancer cells and bloodstream forms of T. b. brucei. The cytotoxic activity of compounds 1u, 1w, 2e and 3d was attributed to their ability to induce apoptosis in cancer cells. The results demonstrate the potential of cinchona alkaloid derivatives as novel anti‐cancer and anti‐trypanosome drug candidates.
中文翻译:

金鸡纳生物碱衍生物的细胞毒性和锥虫活性
使用7种不同的癌细胞系(KB,HeLa,MCF-7,A-549,Hep-G2)对一系列27种金鸡纳生物碱衍生物(1f–w,2a–e和3a–d)的细胞毒性和锥虫活性进行了研究。 ,U‐87和HL‐60),两种正常细胞系(HDF和CHO)和布鲁氏锥虫的血流形式。鉴定出具有前途细胞毒性活性的四种化合物(1u,1w,2e和3d),其50%的生长抑制(GI 50)值低于10μM。四种化合物中的两种(2e和3d)也对GI表现出有效的抗锥虫活性50个值0.3-0.4μM。所有四种活性化合物均代表在其C-9羟基上修饰的衍生物。就抗增殖活性和选择性而言,2e(epi - N-奎尼基-N'-双(3,5-三氟甲基)苯基硫脲)被证明是T细胞的癌细胞和血流形式的最有希望的衍生物。布鲁西。化合物1u,1w,2e和3d的细胞毒活性归因于它们诱导癌细胞凋亡的能力。结果表明金鸡纳生物碱衍生物作为新型抗癌和抗锥虫药物候选物的潜力。
更新日期:2018-07-01
中文翻译:

金鸡纳生物碱衍生物的细胞毒性和锥虫活性
使用7种不同的癌细胞系(KB,HeLa,MCF-7,A-549,Hep-G2)对一系列27种金鸡纳生物碱衍生物(1f–w,2a–e和3a–d)的细胞毒性和锥虫活性进行了研究。 ,U‐87和HL‐60),两种正常细胞系(HDF和CHO)和布鲁氏锥虫的血流形式。鉴定出具有前途细胞毒性活性的四种化合物(1u,1w,2e和3d),其50%的生长抑制(GI 50)值低于10μM。四种化合物中的两种(2e和3d)也对GI表现出有效的抗锥虫活性50个值0.3-0.4μM。所有四种活性化合物均代表在其C-9羟基上修饰的衍生物。就抗增殖活性和选择性而言,2e(epi - N-奎尼基-N'-双(3,5-三氟甲基)苯基硫脲)被证明是T细胞的癌细胞和血流形式的最有希望的衍生物。布鲁西。化合物1u,1w,2e和3d的细胞毒活性归因于它们诱导癌细胞凋亡的能力。结果表明金鸡纳生物碱衍生物作为新型抗癌和抗锥虫药物候选物的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号